J Vet Sci.
2014 Mar;15(1):73-80. 10.4142/jvs.2014.15.1.73.
A simplified one-step nuclear transfer procedure alters the gene expression patterns and developmental potential of cloned porcine embryos
- Affiliations
-
- 1Cellular Reprogramming and Embryo Biotechnology Laboratory, Dental Research Institute, Seoul National University School of Dentistry, Seoul 110-749, Korea. sangho@snu.ac.kr
- 2Department of Theriogenology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. jipark84@konkuk.ac.kr
- KMID: 1737612
- DOI: http://doi.org/10.4142/jvs.2014.15.1.73
Abstract
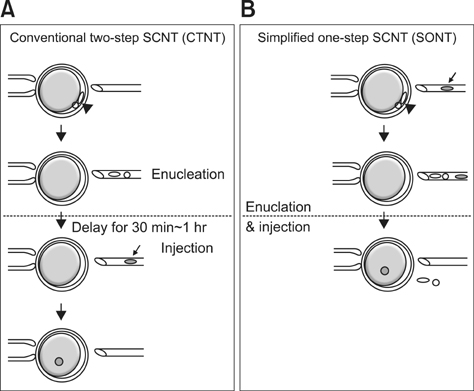
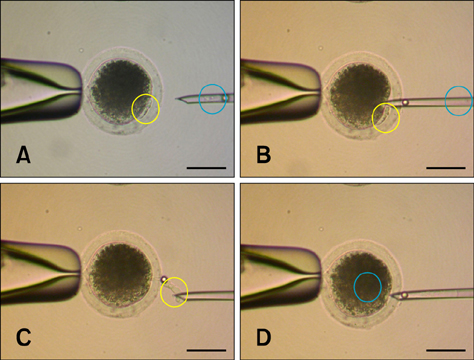
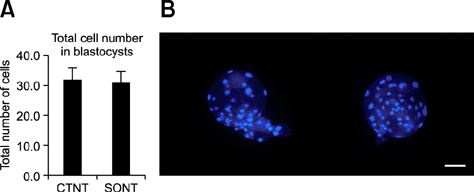
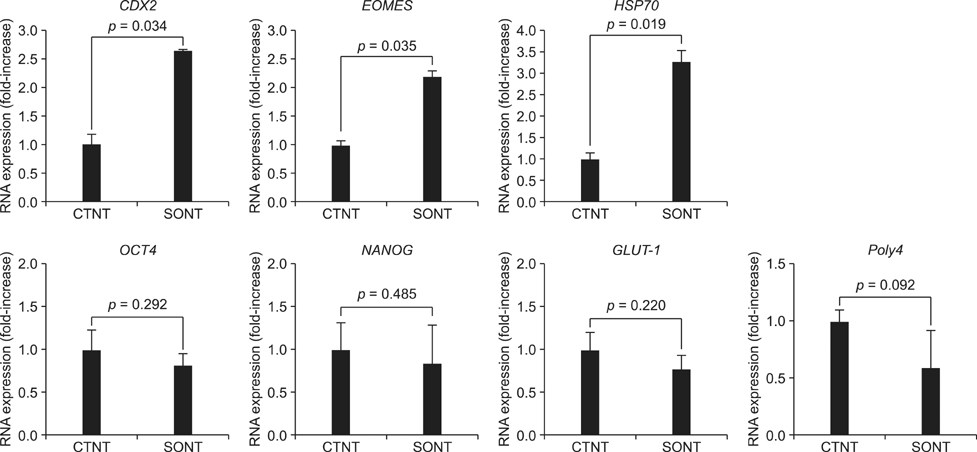
- Various somatic cell nuclear transfer (SCNT) techniques for mammalian species have been developed to adjust species-specific procedures to oocyte-associated differences among species. Species-specific SCNT protocols may result in different expression levels of developmentally important genes that may affect embryonic development and pregnancy. In the present study, porcine oocytes were treated with demecolcine that facilitated enucleation with protruding genetic material. Enucleation and donor cell injection were performed either simultaneously with a single pipette (simplified one-step SCNT; SONT) or separately with different pipettes (conventional two-step SCNT; CTNT) as the control procedure. After blastocysts from both groups were cultured in vitro, the expression levels of developmentally important genes (OCT4, NANOG, EOMES, CDX2, GLUT-1, PolyA, and HSP70) were analyzed by real-time quantitative polymerase chain reaction. Both the developmental rate according to blastocyst stage as well as the expression levels CDX2, EOMES, and HSP70 were elevated with SONT compared to CTNT. The genes with elevated expression are known to influence trophectoderm formation and heat stress-induced arrest. These results showed that our SONT technique improved the development of SCNT porcine embryos, and increased the expression of genes that are important for placental formation and stress-induced arrest.
Keyword
MeSH Terms
Figure
Reference
-
1. Fujimura T, Kurome M, Murakami H, Takahagi Y, Matsunami K, Shimanuki S, Suzuki K, Miyagawa S, Shirakura R, Shigehisa T, Nagashima H. Cloning of the transgenic pigs expressing human decay accelerating factor and N-acetylglucosaminyltransferase III. Cloning Stem Cells. 2004; 6:294–301.
Article2. Prather RS, Sims MM, First NL. Nuclear transplantation in early pig embryos. Biol Reprod. 1989; 41:414–418.3. Won C, Park SK, Cho SG, Min BM, Roh S. Kinetin enhances in vitro development of parthenogenetic and nuclear transfer porcine embryos. Mol Reprod Dev. 2008; 75:1701–1709.
Article4. Li X, Kato Y, Tsuji Y, Tsunoda Y. The effects of trichostatin A on mRNA expression of chromatin structure-, DNA methylation-, and development-related genes in cloned mouse blastocysts. Cloning Stem Cells. 2008; 10:133–142.
Article5. Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007; 22:2232–2242.
Article6. Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005; 14:R47–R58.
Article7. Whitworth KM, Prather RS. Somatic cell nuclear transfer efficiency: how can it be improved through nuclear remodeling and reprogramming? Mol Reprod Dev. 2010; 77:1001–1015.
Article8. Isom SC, Lai L, Prather RS, Rucker EB 3rd. Heat shock of porcine zygotes immediately after oocyte activation increases viability. Mol Reprod Dev. 2009; 76:548–554.
Article9. Kawano K, Kato Y, Tsunoda Y. Comparison of in vitro development of porcine nuclear-transferred oocytes receiving fetal somatic cells by injection and fusion methods. Cloning Stem Cells. 2004; 6:67–72.
Article10. McElroy SL, Kim JH, Kim S, Jeong YW, Lee EG, Park SM, Hossein MS, Koo OJ, Abul Hashem MD, Jang G, Kang SK, Lee BC, Hwang WS. Effects of culture conditions and nuclear transfer protocols on blastocyst formation and mRNA expression in pre-implantation porcine embryos. Theriogenology. 2008; 69:416–425.
Article11. Wrenzycki C, Wells D, Herrmann D, Miller A, Oliver J, Tervit R, Niemann H. Nuclear transfer protocol affects messenger RNA expression patterns in cloned bovine blastocysts. Biol Reprod. 2001; 65:309–317.
Article12. Cervera RP, Silvestre MA, Martí N, García-Mengual E, Moreno R, Stojkovic M. Effects of different oocyte activation procedures on development and gene expression of porcine pre-implantation embryos. Reprod Domest Anim. 2010; 45:e12–e20.
Article13. Costa-Borges N, Paramio MT, Calderón G, Santaló J, Ibáñez E. Antimitotic treatments for chemically assisted oocyte enucleation in nuclear transfer procedures. Cloning Stem Cells. 2009; 11:153–166.
Article14. Costa-Borges N, Paramio MT, Santaló J, Ibáñez E. Demecolcine- and nocodazole-induced enucleation in mouse and goat oocytes for the preparation of recipient cytoplasts in somatic cell nuclear transfer procedures. Theriogenology. 2011; 75:527–541.
Article15. García-Mengual E, Alfonso J, Salvador I, Duque CC, Silvestre MA. Oocyte activation procedures and influence of serum on porcine oocyte maturation and subsequent parthenogenetic and nuclear transfer embryo development. Zygote. 2008; 16:279–284.
Article16. Kurome M, Fujimura T, Murakami H, Takahagi Y, Wako N, Ochiai T, Miyazaki K, Nagashima H. Comparison of electro-fusion and intracytoplasmic nuclear injection methods in pig cloning. Cloning Stem Cells. 2003; 5:367–378.
Article17. Lee JW, Wu SC, Tian XC, Barber M, Hoagland T, Riesen J, Lee KH, Tu CF, Cheng WTK, Yang X. Production of cloned pigs by whole-cell intracytoplasmic microinjection. Biol Reprod. 2003; 69:995–1001.18. Li J, Villemoes K, Zhang Y, Du Y, Kragh PM, Purup S, Xue Q, Pedersen AM, Jørgensen AL, Jakobsen JE, Bolund L, Yang H, Vajta G. Efficiency of two enucleation methods connected to handmade cloning to produce transgenic porcine embryos. Reprod Domest Anim. 2009; 44:122–127.
Article19. Roh S, Choi HY, Park SK, Won C, Kim BW, Kim JH, Kang H, Lee ER, Cho SG. Porcine nuclear transfer using somatic donor cells altered to express male germ cell function. Reprod Fertil Dev. 2009; 21:882–891.
Article20. Yamanaka K, Sugimura S, Wakai T, Kawahara M, Sato E. Difference in sensitivity to culture condition between in vitro fertilized and somatic cell nuclear transfer embryos in pigs. J Reprod Dev. 2009; 55:299–304.
Article21. Galli C, Lagutina I, Vassiliev I, Duchi R, Lazzari G. Comparison of microinjection (piezo-electric) and cell fusion for nuclear transfer success with different cell types in cattle. Cloning Stem Cells. 2002; 4:189–196.
Article22. Yin XJ, Tani T, Yonemura I, Kawakami M, Miyamoto K, Hasegawa R, Kato Y, Tsunoda Y. Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol Reprod. 2002; 67:442–446.
Article23. Zhou Q, Yang SH, Ding CH, He XC, Xie YH, Hildebrandt TB, Mitalipov SM, Tang XH, Wolf DP, Ji WZ. A comparative approach to somatic cell nuclear transfer in the rhesus monkey. Hum Reprod. 2006; 21:2564–2571.
Article24. Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000; 18:1055–1059.
Article25. Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, Cherifi Y, Fraichard A, Cozzi J. Generation of fertile cloned rats by regulating oocyte activation. Science. 2003; 302:1179.
Article26. Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Schöler H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod. 2000; 63:1698–1705.
Article27. Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000; 24:372–376.
Article28. Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005; 132:2093–2102.
Article29. Brevini TAL, Lonergan P, Cillo F, Francisci C, Favetta LA, Fair T, Gandolfi F. Evolution of mRNA polyadenylation between oocyte maturation and first embryonic cleavage in cattle and its relation with developmental competence. Mol Reprod Dev. 2002; 63:510–517.
Article30. Wormington M. Poly(A) and translation: development control. Curr Opin Cell Biol. 1993; 5:950–954.
Article31. Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000; 53:21–34.
Article32. Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998; 92:351–366.
Article33. Cran DG. The distribution of organelles in mammalian oocytes following centrifugation prior to injection of foreign DNA. Gamete Res. 1987; 18:67–76.
Article34. McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JSM, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000; 118:163–170.
Article35. Park SK, Won C, Choi YJ, Kang H, Roh S. The leading blastomere of the 2-cell stage parthenogenetic porcine embryo contributes to the abembryonic part first. J Vet Med Sci. 2009; 71:569–576.
Article36. Vajta G, Maddox-Hyttel P, Skou CT, Tecirlioglu RT, Peura TT, Lai L, Murphy CN, Prather RS, Kragh PM, Callesen H. Highly efficient and reliable chemically assisted enucleation method for handmade cloning in cattle. Reprod Fertil Dev. 2005; 17:791–797.
Article37. Lin J, Shi L, Zhang M, Yang H, Qin Y, Zhang J, Gong D, Zhang X, Li D, Li J. Defects in trophoblast cell lineage account for the impaired in vivo development of cloned embryos generated by somatic nuclear transfer. Cell Stem Cell. 2011; 8:371–375.
Article38. Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008; 135:501–511.
Article39. Parks JC, McCallie BR, Janesch AM, Schoolcraft WB, Katz-Jaffe MG. Blastocyst gene expression correlates with implantation potential. Fertil Steril. 2011; 95:1367–1372.
Article40. Tolkunova E, Cavaleri F, Eckardt S, Reinbold R, Christenson LK, Schöler HR, Tomilin A. The caudal-related protein Cdx2 promotes trophoblast differentiation of mouse embryonic stem cells. Stem Cells. 2006; 24:139–144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of porcine urine-derived cells as nuclei donor for somatic cell nuclear transfer
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes
- Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos
- Cloned calves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid
- Improved preimplantation development of porcine somatic cell nuclear transfer embryos by caffeine treatment