J Vet Sci.
2014 Mar;15(1):51-60. 10.4142/jvs.2014.15.1.51.
Protective effects of silymarin on fumonisin B1-induced hepatotoxicity in mice
- Affiliations
-
- 1Department of Pathology, Samsun, Faculty of Veterinary Medicine, University of Ondokuz Mayis, Samsun 55139, Turkey. msozmen@hotmail.com
- 2Department of Biochemistry, Faculty of Veterinary Medicine, University of Mehmet Akif Ersoy, Burdur 15100, Turkey.
- 3Department of Pathology, Faculty of Veterinary Medicine, University of Adnan Menderes, Aydin 09010, Turkey.
- 4Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Mehmet Akif Ersoy, Burdur 15100, Turkey.
- 5Department of Pathology, Faculty of Veterinary Medicine, University of Kafkas, Kars 36100, Turkey.
- 6Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Kirikkale, Kirikkale 71450, Turkey.
- KMID: 1737610
- DOI: http://doi.org/10.4142/jvs.2014.15.1.51
Abstract
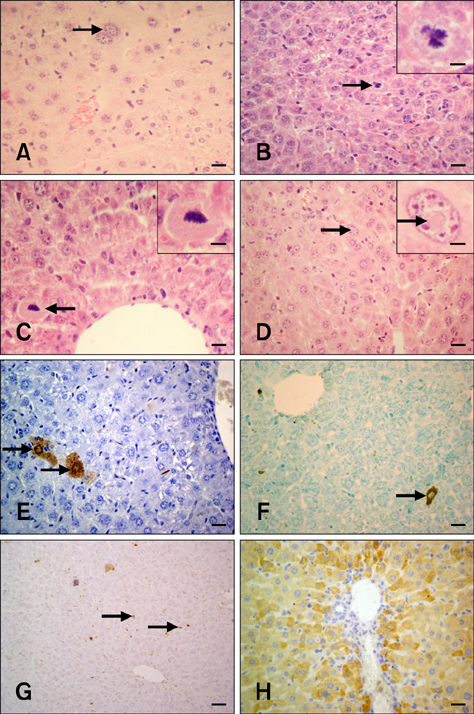
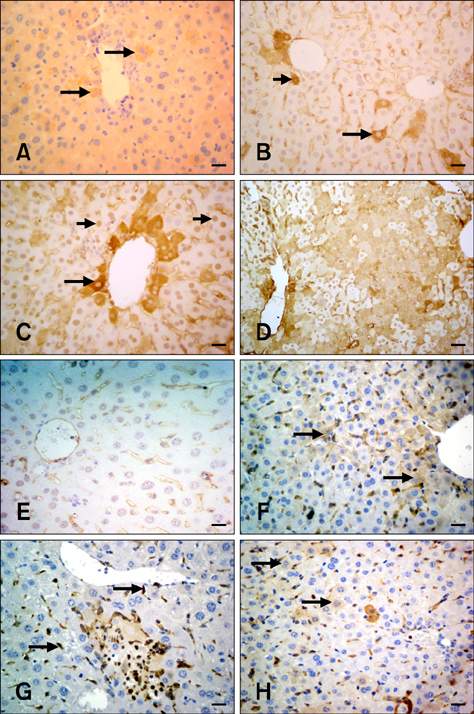
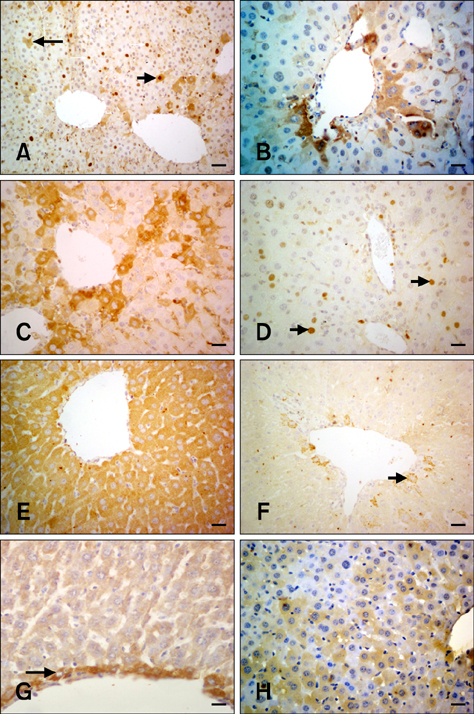
- The present study was conducted to investigate the effect of silymarin on experimental liver toxication induced by Fumonisin B1 (FB1) in BALB/c mice. The mice were divided into six groups (n = 15). Group 1 served as the control. Group 2 was the silymarin control (100 mg/kg by gavage). Groups 3 and 4 were treated with FB1 (Group 3, 1.5 mg/kg FB1, intraperitoneally; and Group 4, 4.5 mg/kg FB1). Group 5 received FB1 (1.5 mg/kg) and silymarin (100 mg/kg), and Group 6 was given a higher dose of FB1 (4.5 mg/kg FB1) with silymarin (100 mg/kg). Silymarin treatment significantly decreased (p < 0.0001) the apoptotic rate. FB1 administration significantly increased (p < 0.0001) proliferating cell nuclear antigen and Ki-67 expression. Furthermore, FB1 elevated the levels of caspase-8 and tumor necrosis factor-alpha mediators while silymarin significantly reduced (p < 0.0001) the expression of these factors. Vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) expressions were significantly elevated in Group 4 (p < 0.0001). Silymarin administration alleviated increased VEGF and FGF-2 expression levels (p < 0.0001). In conclusion, silymarin ameliorated toxic liver damage caused by FB1 in BALB/c mice.
MeSH Terms
-
Animals
Antioxidants/*pharmacology
Apoptosis/drug effects
Cell Proliferation/drug effects
Female
Fibroblast Growth Factor 2/genetics/metabolism
Fumonisins/*toxicity
Gene Expression Regulation/drug effects
Hepatocytes/*drug effects
Ki-67 Antigen/metabolism
Liver/drug effects
Mice
Mice, Inbred BALB C
Mycotoxins/*toxicity
Neovascularization, Physiologic/drug effects
Proliferating Cell Nuclear Antigen/metabolism
Silymarin/*pharmacology
Tumor Necrosis Factor-alpha/metabolism
Vascular Endothelial Growth Factor A/genetics/metabolism
Antioxidants
Fibroblast Growth Factor 2
Fumonisins
Ki-67 Antigen
Mycotoxins
Proliferating Cell Nuclear Antigen
Silymarin
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Ankoma-Sey V, Matli M, Chang KB, Lalazar A, Donner DB, Wong L, Warren RS, Friedman SL. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene. 1998; 17:115–121.
Article2. Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992; 59:115–165.
Article3. Bhandari N, He Q, Sharma RP. Gender-related differences in subacute fumonisin B1 hepatotoxicity in BALB/c mice. Toxicology. 2001; 165:195–204.
Article4. Bhandari N, Sharma RP. Fumonisin B1-induced alterations in cytokine expression and apoptosis signaling genes in mouse liver and kidney after an acute exposure. Toxicology. 2002; 172:81–92.
Article5. Bhandari N, Sharma RP. Modulation of selected cell signaling genes in mouse liver by fumonisin B1. Chem Biol Interact. 2002; 139:317–331.
Article6. Ciacci-Zanella JR, Jones C. Fumonisin B1, a mycotoxin contaminant of cereal grains and inducer of apoptosis via the tumor necrosis factor pathway and caspase activation. Food Chem Toxicol. 1999; 37:703–712.
Article7. Donahower B, Mc Cullough SS, Kurten R, Lamps LW, Simpson P, Hinson JA, James LP. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G102–G109.
Article8. Dragan YP, Bidlack WR, Cohen SM, Goldsworthy TL, Hard GC, Howard PC, Riley RT, Voss KA. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: fumonisin B1 as an example. Toxicol Sci. 2001; 61:6–17.
Article9. Dugyala RR, Sharma RP, Tsunoda M, Riley RT. Tumor necrosis factor-α as a contributor in fumonisin B1-toxicity. J Pharmacol Exp Ther. 1998; 285:317–324.10. He Q, Kim J, Sharma RP. Silymarin protects against liver damage in BALB/c mice exposed to fumonisin B1 despite increasing accumulation of free sphingoid bases. Toxicol Sci. 2004; 80:335–342.
Article11. Hsu DK, Dowling CA, Jeng KCG, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999; 81:519–526.
Article12. Hsu YC, Chiu YT, Lee CY, Wu CF, Huang YT. Anti-fibrotic effects of tetrandrine on bile-duct ligated rats. Can J Physiol Pharmacol. 2006; 84:967–976.
Article13. Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, Ikeda H, Ohno A, Shibuya M, Fujiwara K. Expressions of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun. 1999; 254:587–593.
Article14. Kim H, Lee J, Hyun JW, Park JW, Joo H, Shin T. Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol Int. 2007; 31:655–662.
Article15. Li LH, Wu LJ, Tashiro S, Onodera S, Uchiumi F, Ikejima T. Silibinin prevents UV-induced HaCaT cell apoptosis partly through inhibition of caspase-8 pathway. Biol Pharm Bull. 2006; 29:1096–1101.
Article16. Lim CW, Parker HM, Vesonder RF, Haschek WM. Intravenous fumonisin B1 induces cell proliferation and apoptosis in the rat. Nat Toxins. 1996; 4:34–41.
Article17. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002; 1572:263–273.
Article18. Merrill AH Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Sphingolipids-the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997; 142:208–225.
Article19. Rastogi R, Srivastava AK, Srivastava M, Rastogi AK. Hepatocurative effect of picroliv and silymarin against aflatoxin B1 induced hepatotoxicity in rats. Planta Med. 2000; 66:709–713.
Article20. Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999; 155:1065–1073.
Article21. Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003; 39:333–340.
Article22. Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K, Hirabayashi J, Kasai K, Nakanuma Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001; 32:302–310.
Article23. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359:843–845.
Article24. Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001; 49:121–129.
Article25. Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006; 168:695–705.
Article26. Voss KA, Riley RT, Bacon CW, Chamberlain WJ, Norred WP. Subchronic toxic effects of Fusarium moniliforme and fumonisin B1 in rats and mice. Nat Toxins. 1996; 4:16–23.
Article27. WHO IPCS. Fumonisin B1. Environmental Health Criteria 219. Geneva: World Health Organization;2000. p. 1–150.28. Yamazaki K, Kawai A, Kawaguchi M, Hibino Y, Li F, Sasahara M, Tsukada K, Hiraga K. Simultaneous induction of galectin-3 phosphorylated on tyrosine residue, p21WAF1/Cip1/Sdi1, and the proliferating cell nuclear antigen at a distinctive period of repair of hepatocytes injured by CCl4. Biochem Biophys Res Commun. 2001; 280:1077–1084.
Article29. Yang SH, Lin JK, Huang CJ, Chen WS, Li SY, Chiu JH. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation. J Surg Res. 2005; 128:140–146.
Article30. Zi X, Mukhtar H, Agarwal R. Novel cancer chemopreventive effects of a flavonoid antioxidant silymarin: inhibition of m RNA expression of an endogenous tumor promoter TNFα. Biochem Biophys Res Commun. 1997; 239:334–339.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Prophylactic Use of Silymarin on Anti-tuberculosis Drugs Induced Hepatotoxicity
- Anti-oxidant activities of kiwi fruit extract on carbon tetrachloride-induced liver injury in mice
- Fumonisin B1-Induced Toxicity Was Not Exacerbated in Glutathione Peroxidase-1/Catalase Double Knock Out Mice
- Protective Effect of Cholesteryl Hemisuccinate on Fumonisin B1-nduced Apoptosis of Hepatocytes in the Rat Liver
- Early ultrastructural changes of apoptosis induced by fumonisin B1 in rat liver