J Vet Sci.
2014 Mar;15(1):35-43. 10.4142/jvs.2014.15.1.35.
Immunohistochemical evaluation of the goat forestomach during prenatal development
- Affiliations
-
- 1Department of Veterinary Histology, Faculty of Veterinary Medicine, University of Extremadura, 10071 Caceres, Spain. eloy@unex.es
- 2Department of Veterinary Anatomy, Faculty of Veterinary Medicine, University of Extremadura, 10071 Caceres, Spain.
- KMID: 1737608
- DOI: http://doi.org/10.4142/jvs.2014.15.1.35
Abstract
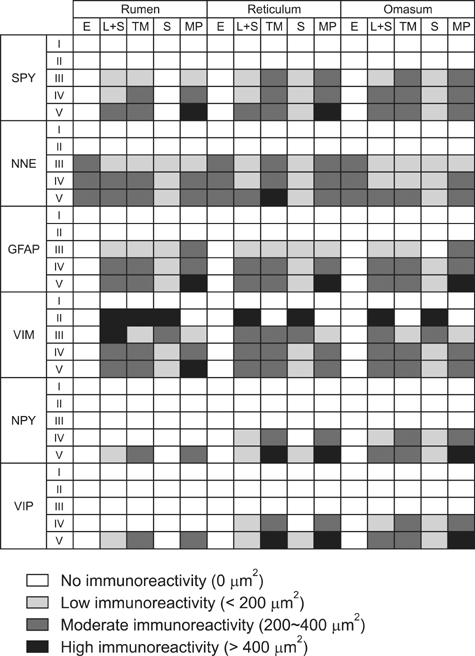
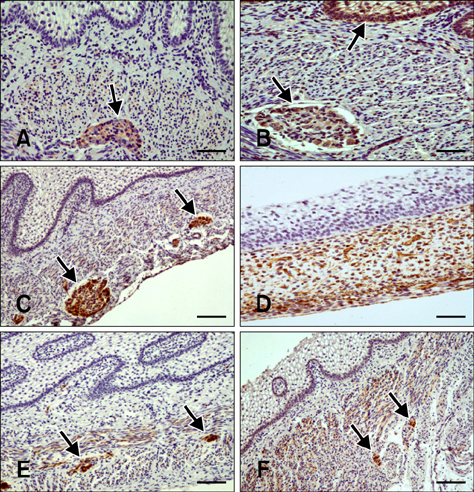
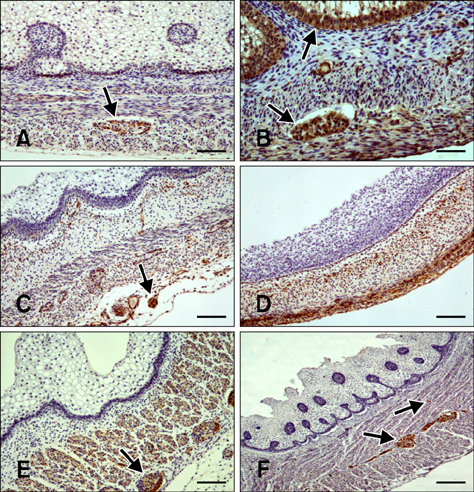
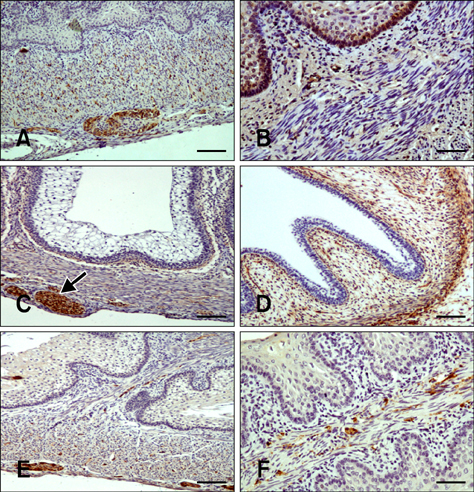
- Here we report the detection and distribution of synaptophysin (SPY), non-neuronal enolase (NNE), glial fibrillary acidic protein (GFAP), vimentin (VIM), neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) expression in the goat forestomach during prenatal development. A total of 140 embryos and fetuses were examined to evaluate protein expression from the first stage of prenatal life until birth. In all cases, SPY immunoreactivity was detected at 53 days gestation in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexuses. Immunoreactivity to NNE was observed at 64 days gestation in the same locations as well as the epithelial layer. Glial cells were found at 64 days as indicated by signals corresponding to GFAP and VIM at 39 days. Positive staining for NPY and VIP was observed at 113, 75, and 95 days in the rumen, reticulum, and omasum, respectively, in the lamina propria-submucosa, tunica muscularis, and myenteric plexuses of each of these gastric compartments. These findings indicate possible preparation of the fetal goat forestomach for postnatal function. Compared to other ruminant species, neuroendocrine cells, glial cells and peptidergic innervations markers were detected earlier compared to sheep but at around the same stage as in deer.
Keyword
MeSH Terms
-
Animals
Biological Markers/metabolism
Embryo, Mammalian
Endocrine Cells/*metabolism
Fetus/metabolism
Gene Expression Regulation, Developmental
Goats/*embryology/genetics
Immunohistochemistry
Neuroendocrine Cells/*metabolism
Neuroglia/*metabolism
Proteins/genetics
Rumen/*embryology/metabolism
Biological Markers
Proteins
Figure
Reference
-
1. Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M, Lardeux B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 2010; 24:1082–1094.
Article2. Calingasan NY, Kitamura N, Yamada J, Oomori Y, Yamashita T. Immunocytochemical study of the gastroenteropancreatic endocrine cells of the sheep. Acta Anat. 1984; 118:171–180.
Article3. Ceccarelli P, Pedini V, Gargiulo AM. Enteroendocrine cells in sheep fetuses. Small Rumin Res. 1991; 6:85–93.
Article4. Deveney CW, Way WL. Regulatory peptides of the gut. In : Greenspan FS, Forsham PH, editors. Basic and Clinical Endocrinology. Asian ed. Singapore: Maruzen;1983. p. 479–499.5. Evans HE, Sack WO. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralbl Veterinarmed C. 1973; 2:11–45.
Article6. Franco A, Masot AJ, Redondo E. Ontogenesis of the rumen: a comparative analysis of the merino sheep and Iberian red deer. Anim Sci J. 2011; 82:107–116.
Article7. Franco A, Masot J, García A, Redondo E. Ontogenesis of the reticulum with special reference to neuroendocrine and glial cells: a comparative analysis of the merino sheep and Iberian red deer. Anat Histol Embryol. 2012; 41:362–373.
Article8. Franco A, Regodon S, Masot AJ, Redondo E. A combined immunohistochemical and electron microscopic study of the second cell type in the developing sheep pineal gland. J Pineal Res. 1997; 22:130–136.
Article9. Franco AJ, Masot AJ, Aguado MC, Gómez L, Redondo E. Morphometric and immunohistochemical study of the rumen of red deer during prenatal development. J Anat. 2004; 204:501–513.
Article10. Franco AJ, Redondo E, Masot AJ. Morphometric and immunohistochemical study of the reticulum of red deer during prenatal development. J Anat. 2004; 205:277–289.
Article11. Garcia A, Masot J, Franco A, Gazquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat omasum during prenatal development. Histol Histopathol. 2013; 28:737–748.12. Garcia A, Masot J, Franco A, Gazquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat reticulum during prenatal development. Histol Histopathol. 2013; 28:1369–1381.13. García A, Masot J, Franco A, Gázquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat rumen during prenatal development. Anat Rec (Hoboken). 2012; 295:776–785.
Article14. Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In : Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3rd ed. New York: Raven Press;1994. p. 381–422.15. Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black Karakul lambs. Onderstepoort J Vet Res. 1994; 61:207–213.16. Kitamura N, Yamada J, Yamamoto Y, Yamashita J. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch Histol Cytol. 1993; 56:399–410.
Article17. Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase- and peptide-containing nerves in the reticulorumen and the reticular groove of cattle. J Comp Neurol. 1986; 248:223–234.
Article18. Kleinschmidt S, Nolte I, Hewicher-Trautwein M. Structural and functional components of the feline enteric nervous system. Anat Histol Embryol. 2011; 40:450–456.
Article19. Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980; 283:249–256.
Article20. Lombardi G. Optimum management and quality pastures for sheep and goat in mountain areas. Options Méditerranéennes. 2005; 67:19–29.21. Münnich J, Gäbel G, Pfannkuche H. Intrinsic ruminal innervation in ruminants of different feeding types. J Anat. 2008; 213:442–451.
Article22. Nada O, Kawana T. Immunohistochemical identification of supportive cell types in the enteric nervous system of the rat colon and rectum. Cell Tissue Res. 1988; 251:523–529.
Article23. Pan QS, Fang ZP, Huang FJ. Identification, lozalization and morphology of APUD cell in gastroenteropan creatic system of stomach-containing teleosts. World J Gastroenterol. 2000; 6:842–847.
Article24. Pearse AGE, Takor TT. Neuroendocrine embryology and the APUD concept. Clin Endocrinol (Oxf). 1976; 5:Suppl. 229S–244S.
Article25. Pfannkuche H, Schellhorn C, Schemann M, Gäbel G. Intrinsic innervation patterns of the smooth muscle in the rumen and reticulum of lambs. J Anat. 2004; 204:293–299.
Article26. Pfannkuche H, Schellhorn C, Schemann M, Gäbel G. Reticular groove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat Rec A Discov Mol Cell Evol Biol. 2003; 274:917–922.
Article27. Pfannkuche H, Schemann M, Gäbel G. Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res. 2002; 309:347–354.
Article28. Redondo E, Franco AJ, Masot AJ. Morphometric and immunohistochemical study of the omasum of red deer during prenatal development. J Anat. 2005; 206:543–555.
Article29. Redondo E, Masot J, García A, Franco A. Ontogenesis of the omasum: a comparative analysis of the merino sheep and Iberian red deer. Histol Histopathol. 2011; 26:1135–1144.30. Ruckebusch Y. Gastrointestinal motor functions in ruminants. In : Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology. The Gastrointestinal System. New York: Oxoford University Press;1989. Vol. 1:p. 1225–1283.31. Schummer A, Nickel R. Lehrbuch der Anatomie der Haustiere: Eingeweide. 3rd ed. Parey: Berlin;1975. Vol. 2:p. 149–173.32. Sidhu GS. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979; 96:5–20.33. Sivachelvan MN, Ali MG, Chibuzo GA. Foetal age estimation in sheep and goats. Small Rumin Res. 1996; 19:69–76.
Article34. Teixeira AF, Wedel T, Krammer HJ, Kühnel W. Structural differences of the enteric nervous system in the cattle forestomach revealed by whole mount immunohistochemistry. Ann Anat. 1998; 180:393–400.
Article35. Titchen DA. Nervous control of motility of the forestomach of ruminant. In : Heidel W, Code CF, editors. Handbook of Physiology: Alimentary Canal. Baltimore: Waverly Press;1968. Vol. 5:p. 2705–2724.36. Von Boyen GBT, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004; 53:222–228.
Article37. Wathuta EM, Harrison FA. The ontogeny of vasoactive intestinal polypeptide-like and substance P-like immunoreactivity in the digestive tract of the sheep. Q J Exp Physiol. 1987; 72:119–128.
Article38. Yamamoto Y, Kitamura N, Yamada J, Yamashita T. Immunoohistochemical study of the distributions of the peptide- and cathecholamine-containing nerves in the omasum of the sheep. Acta Anat. 1994; 149:104–110.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical localization of neuron specific enolase in developing tongue of Korean native goat (Capra hircus)
- Immunohistochemical localization of calcium binding proteins and some neurotransmitters in myenteric plexus of goat stomach
- Double Immunohistochemical Studies on Distribution and Coexistence with Putative Neurotransmitters and Calretinin in Trigeminal Ganglion of Korean Native Goat
- Immunohistochemical study on cytokeratin expression on developing tongue in Korean native goats (Capra hircus)
- Immunohistochemical study of constitutive neuronal and inducible nitric oxide synthase in the central nervous system of goat with natural listeriosis