J Korean Med Sci.
2014 Mar;29(3):378-385. 10.3346/jkms.2014.29.3.378.
Simulation of Oral Glucose Tolerance Tests and the Corresponding Isoglycemic Intravenous Glucose Infusion Studies for Calculation of the Incretin Effect
- Affiliations
-
- 1Interdisciplinary Program for Bioengineering, Graduate School, Seoul National University, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Medical and Biological Engineering, Medical Research Center, Seoul National University, Seoul, Korea. sungwan@snu.ac.kr
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 5Department of Biomedical Engineering, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Biomedical Engineering, Seoul National University Hospital, Seoul, Korea.
- KMID: 1734925
- DOI: http://doi.org/10.3346/jkms.2014.29.3.378
Abstract
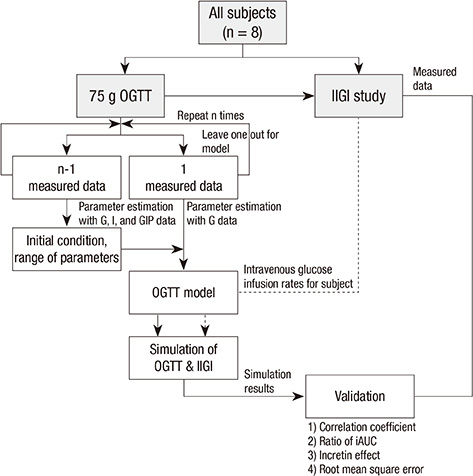
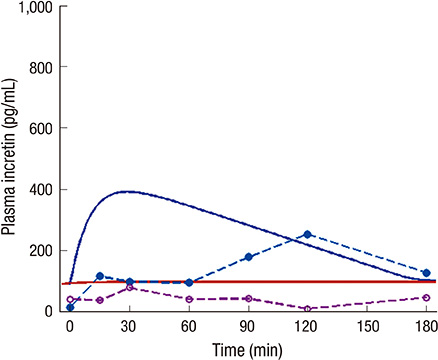
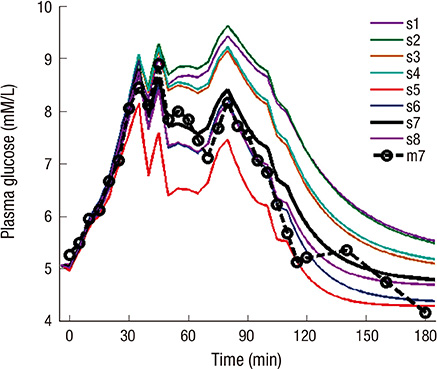
- The incretin effect, which is a unique stimulus of insulin secretion in response to oral ingestion of nutrients, is calculated by the difference in insulin secretory responses from an oral glucose tolerance test (OGTT) and a corresponding isoglycemic intravenous glucose infusion (IIGI) study. The OGTT model of this study, which is individualized by fitting the glucose profiles during an OGTT, was developed to predict the glucose profile during an IIGI study in the same subject. Also, the model predicts the insulin and incretin profiles during both studies. The incretin effect, estimated by simulation, was compared with that measured by physiologic studies from eight human subjects with normal glucose tolerance, and the result exhibited a good correlation (r > 0.8); the incretin effect from the simulation was 56.5% +/- 10.6% while the one from the measured data was 52.5% +/- 19.6%. In conclusion, the parameters of the OGTT model have been successfully estimated to predict the profiles of both OGTTs and IIGI studies. Therefore, with glucose data from the OGTT alone, this model could control and predict the physiologic responses, including insulin secretion during OGTTs and IIGI studies, which could eventually eliminate the need for complex and cumbersome IIGI studies in incretin research.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
Endocrinol Metab. 2023;38(1):1-9. doi: 10.3803/EnM.2023.103.
Reference
-
1. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986; 29:46–52.2. Nauck MA, El-Ouaghlidi A, Gabrys B, Hücking K, Holst JJ, Deacon CF, Gallwitz B, Schmidt WE, Meier JJ. Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul Pept. 2004; 122:209–217.3. Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986; 63:492–498.4. Knop FK, Vilsbøll T, Højberg PV, Larsen S, Madsbad S, Vølund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007; 56:1951–1959.5. McIntosh CH, Widenmaier S, Kim SJ. Pleiotropic actions of the incretin hormones. Vitam Horm. 2010; 84:21–79.6. Dedík L, Durisová M, Penesová A, Miklovicová D, Tvrdonová M. Estimation of influence of gastric emptying on shape of glucose concentration-time profile measured in oral glucose tolerance test. Diabetes Res Clin Pract. 2007; 77:377–384.7. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004; 287:E637–E643.8. Thomaseth K, Pavan A, Berria R, Glass L, DeFronzo R, Gastaldelli A. Model-based assessment of insulin sensitivity of glucose disposal and endogenous glucose production from double-tracer oral glucose tolerance test. Comput Methods Programs Biomed. 2008; 89:132–140.9. Chee F, Fernando T. Closed-loop control of blood glucose. Berlin; New York: Springer;2007. p. 157.10. Salinari S, Bertuzzi A, Mingrone G. Intestinal transit of a glucose bolus and incretin kinetics: a mathematical model with application to the oral glucose tolerance test. Am J Physiol Endocrinol Metab. 2011; 300:E955–E965.11. Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes. 2007; 56:1489–1501.12. Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008; 57:678–687.13. Brubaker PL, Ohayon EL, D'Alessandro LM, Norwich KH. A mathematical model of the oral glucose tolerance test illustrating the effects of the incretins. Ann Biomed Eng. 2007; 35:1286–1300.14. Overgaard RV, Jelic K, Karlsson M, Henriksen JE, Madsen H. Mathematical beta cell model for insulin secretion following IVGTT and OGTT. Ann Biomed Eng. 2006; 34:1343–1354.15. Burattini R, Morettini M. Identification of an integrated mathematical model of standard oral glucose tolerance test for characterization of insulin potentiation in health. Comput Methods Programs Biomed. 2012; 107:248–261.16. Fred A, Filipe J, Gamboa H. In : Biomedical engineering systems and technologies : third International Joint Conference, BIOSTEC 2010; January 20-23, 2010; Valencia, Spain. Heidelberg; Berlin; New York: Springer;2011. p. 408. revised selected papers.17. Hovorka R, Chassin LJ, Ellmerer M, Plank J, Wilinska ME. A simulation model of glucose regulation in the critically ill. Physiol Meas. 2008; 29:959–978.18. Muscelli E, Mari A, Natali A, Astiarraga BD, Camastra S, Frascerra S, Holst JJ, Ferrannini E. Impact of incretin hormones on beta-cell function in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2006; 291:E1144–E1150.19. Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996; 97:92–103.20. Anderwald C, Gastaldelli A, Tura A, Krebs M, Promintzer-Schifferl M, Kautzky-Willer A, Stadler M, DeFronzo RA, Pacini G, Bischof MG. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. 2011; 96:515–524.21. Yu SH, Cho B, Lee Y, Kim E, Choi SH, Lim S, Yi KH, Park YJ, Park KS, Jang HC. Insulin secretion and incretin hormone concentration in women with previous gestational diabetes mellitus. Diabetes Metab J. 2011; 35:58–64.22. Azuma K, Rádiková Z, Mancino J, Toledo FG, Thomas E, Kangani C, Dalla Man C, Cobelli C, Holst JJ, Deacon CF, et al. Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008; 93:459–464.23. Vardarli I, Nauck MA, Köthe LD, Deacon CF, Holst JJ, Schweizer A, Foley JE. Inhibition of DPP-4 with vildagliptin improved insulin secretion in response to oral as well as "isoglycemic" intravenous glucose without numerically changing the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011; 96:945–954.24. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007; 132:2131–2157.25. Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003; 114:115–121.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vivo Models for Incretin Research: From the Intestine to the Whole Body
- Incretin Hormones: Pathophysiological Risk Factors and Potential Targets for Type 2 Diabetes
- The effect of regular physical exercise on glucose uptake in soleus and intravenous glucose tolerance in streptozotocin diabetic rats
- Continuous Intravenous Glucose Infusion and Serum Glucose in Neonates
- One Cases of Trinsient Neonatal Diabetes