J Korean Soc Transplant.
2014 Sep;28(3):113-120. 10.4285/jkstn.2014.28.3.113.
Integration of the Innate and Adaptive Immunity by CD137-CD137L Bidirectional Signals: Implications in Allograft Rejection
- Affiliations
-
- 1Department of Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. hrcho@uuh.ulsan.kr
- 2Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 3Biomedical Research Center, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. bkwon@mail.ulsan.ac.kr
- 4School of Biological Sciences, University of Ulsan, Ulsan, Korea.
- KMID: 1727515
- DOI: http://doi.org/10.4285/jkstn.2014.28.3.113
Abstract
- Two-signal models are useful in explaining various types of immune responses. In particular, secondary, so-called costimulatory, signals are critically required for the process of T-cell activation, survival, differentiation, and memory formation. Early studies in rodent models showed that targeting T-cell costimulatory pathways elicits immunological tolerance, providing a basis for development of costimulatory therapeutics in allograft rejection. However, as the classic definition of T-cell costimulation continues to evolve, simple blockade of costimulatory pathways has limitations in prevention of allograft rejection. Furthermore, functions of costimulatory molecules are much more diverse than initially anticipated and beyond T cells. In this mini-review, we will discuss CD137-CD137L bidirectional signals as examples showing that two-signals can be applicable to multiple phases of immune responses.
Keyword
Figure
Reference
-
References
1). Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002; 2:439–46.
Article2). Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Semin Immunol. 2011; 23:293–303.
Article3). Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010; 11:21–27.
Article4). Wekerle T, Kurtz J. Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002; 14:592–600.5). Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997; 94:8789–94.
Article6). Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999; 5:686–93.
Article7). Xu H, Tadaki DK, Elster EA, Burkly LC, Berning JD, Cruzata F, et al. Humanized anti-CD154 antibody therapy for the treatment of allograft rejection in nonhuman primates. Transplantation. 2002; 74:940–3.
Article8). Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in nonhuman primates: progress, current challenges and unmet needs. Am J Transplant. 2006; 6(5 Pt 1):884–93.
Article9). Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for longterm maintenance immunosuppression. Am J Transplant. 2006; 6(5 Pt 1):876–83.
Article10). Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002; 2:501–9.
Article11). Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009; 229:294–306.
Article12). Chen L, Flies DB. Molecular mechanisms of T cell costimulation and co-inhibition. Nat Rev Immunol. 2013; 13:227–42.
Article13). Fontana MF, Vance RE. Two signal models in innate immunity. Immunol Rev. 2011; 243:26–39.
Article14). Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009; 9:271–85.
Article15). Kwon B. CD137-CD137 Ligand Interactions in Inflammation. Immune Netw. 2009; 9:84–9.
Article16). Lee EA, Kim JE, Seo JH, Kwon BS, Nam SH, Kwon B, et al. 4–1BB (CD137) signals depend upon CD28 signals in alloimmune responses. Exp Mol Med. 2006; 38:606–15.
Article17). Wang J, Guo Z, Dong Y, Kim O, Hart J, Adams A, et al. Role of 4–1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant. 2003; 3:543–51.18). Nozawa K, Ohata J, Sakurai J, Hashimoto H, Miyajima H, Yagita H, et al. Preferential blockade of CD8 (+) T cell responses by administration of anti-CD137 ligand monoclonal antibody results in differential effect on development of murine acute and chronic graft-versus-host diseases. J Immunol. 2001; 167:4981–6.19). Kim J, Choi WS, Kang H, Kim HJ, Suh JH, Sakaguchi S, et al. Conversion of alloantigen-specific CD8+ T cell anergy to CD8+ T cell priming through in vivo ligation of gluco-corticoid-induced TNF receptor. J Immunol. 2006; 176:5223–31.20). Via CS. Advances in lupus stemming from the parent-in-to-F1 model. Trends Immunol. 2010; 31:236–45.
Article21). Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4–1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001; 166:3174–83.
Article22). Lee SC, Seo KW, Kim HJ, Kang SW, Choi HJ, Kim A, et al. Depletion of alloreactive T cells by anti-CD137-saporin immunotoxin. Cell Transplant [in press 2014 Mar 3].23). Cho HR, Kwon B, Yagita H, La S, Lee EA, Kim JE, et al. Blockade of 4-1BB (CD137)/4–1BB ligand interactions increases allograft survival. Transpl Int. 2004; 17:351–61.
Article24). Kwon B. Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease. Exp Mol Med. 2010; 42:675–83.
Article25). Kim W, Kim J, Jung D, Kim H, Choi HJ, Cho HR, et al. Induction of lethal graft-versus-host disease by anti-CD137 monoclonal antibody in mice prone to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2009; 15:306–14.
Article26). Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, et al. Stimulation with 4–1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005; 105:2206–13.27). Kim J, Kim HJ, Park K, Kim J, Choi HJ, Yagita H, et al. Costimulatory molecule-targeted immunotherapy of cutaneous graft-versus-host disease. Blood. 2007; 110:776–82.
Article28). Kim J, Cho HR, Kwon B. Anti-CD137 mAb Deletes Both Donor CD4 and CD8 T Cells in Acute Graft-versus-host Disease. Immune Netw. 2011; 11:428–30.29). Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013; 12:147–68.
Article30). Kwon B. Regulation of Inflammation by Bidirectional Signaling through CD137 and Its Ligand. Immune Netw. 2012; 12:176–80.
Article31). Park SJ, Kim HJ, Lee JS, Cho HR, Kwon B. Reverse signaling through the costimulatory ligand, CD137L, as a critical mediator of sterile inflammation. Mol Cells. 2012; 33:533–7.
Article32). Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamo-to M, Kinoshita T, et al. Costimulation of mast cells by 4–1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood. 2005; 106:4241–8.
Article33). Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, et al. Stimulation of the molecule 4–1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect Immun. 2009; 77:2168–76.34). Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4–1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012; 189:2697–701.
Article35). Nguyen QT, Nguyen TH, Ju SA, Lee YS, Han SH, Lee SC, et al. CD137 expressed on neutrophils plays dual roles in antibacterial responses against Gram-positive and Gram-negative bacterial infections. Infect Immun. 2013; 81:2168–77.
Article36). Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, et al. Stimulation of the molecule 4–1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect Immun. 2009; 77:2168–76.37). Lee SC, Ju SA, Pack HN, Heo SK, Suh JH, Park SM, et al. 4–1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun. 2005; 73:5144–51.38). Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, et al. CD137 (4–1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010; 121:1124–33.
Article39). Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, et al. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J. 2007; 21:456–63.
Article40). Jung IH, Choi JH, Jin J, Jeong SJ, Jeon S, Lim C, et al. CD137-inducing factors from T cells and macrophages accelerate the destabilization of atherosclerotic plaques in hyperlipidemic mice. FASEB J [in press 2014 Jul 24].41). Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011; 89:21–9.
Article42). Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, et al. Cell surface 4–1BBL mediates sequential signaling pathways ‘downstream' of TLR and is required for sustained TNF production in macrophages. Nat Immunol. 2007; 8:601–9.
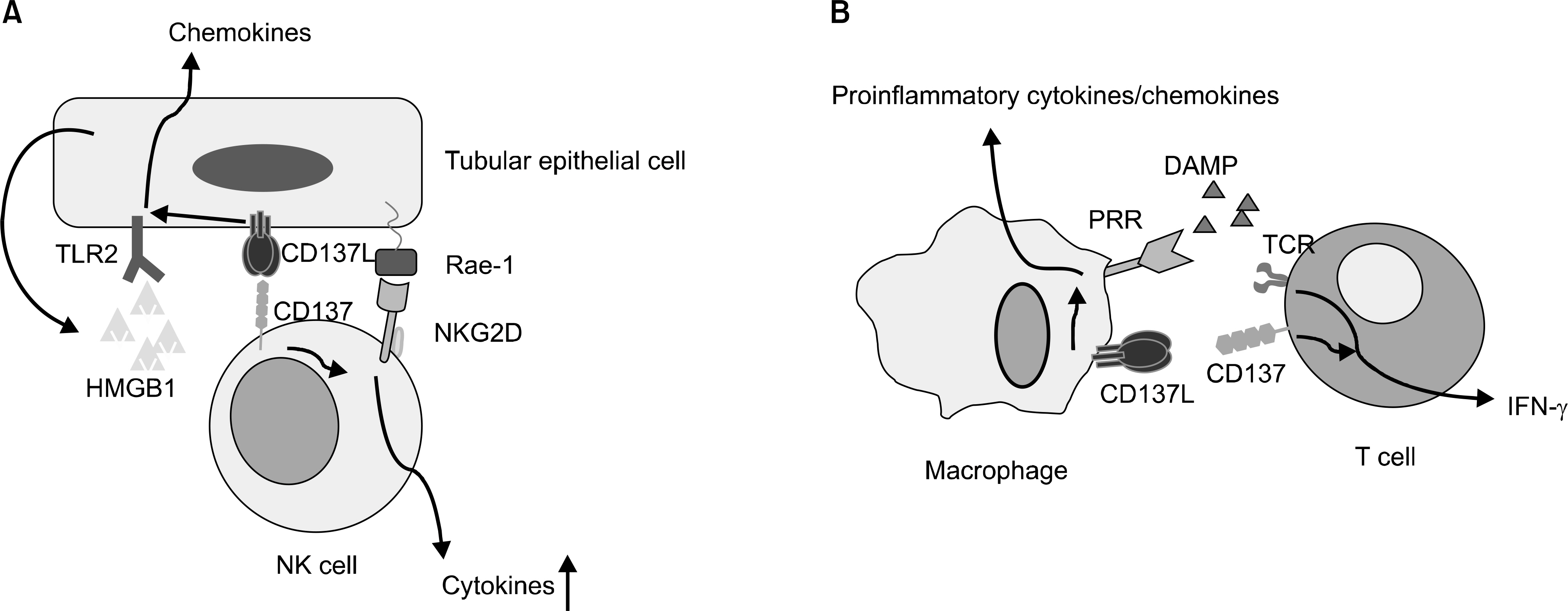
Article43). Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, et al. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc Natl Acad Sci USA. 2012; 109:E13–22.
Article44). Kim HJ, Lee JS, Kim A, Koo S, Cha HJ, Han JA, et al. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury. J Immunol. 2013; 191:2657–64.
Article45). Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008; 181:7489–98.
Article