J Bacteriol Virol.
2014 Sep;44(3):261-268. 10.4167/jbv.2014.44.3.261.
Uncleaved Dystrophin Induce Cardiac Myocyte Apoptosis in Coxsackievirus Infected Balb/C Background Mice Heart
- Affiliations
-
- 1Department of Biomedical Science, Jungwon University, Goesan-gun, Chungbuk, Korea. bklim@jwu.ac.kr
- KMID: 1726976
- DOI: http://doi.org/10.4167/jbv.2014.44.3.261
Abstract
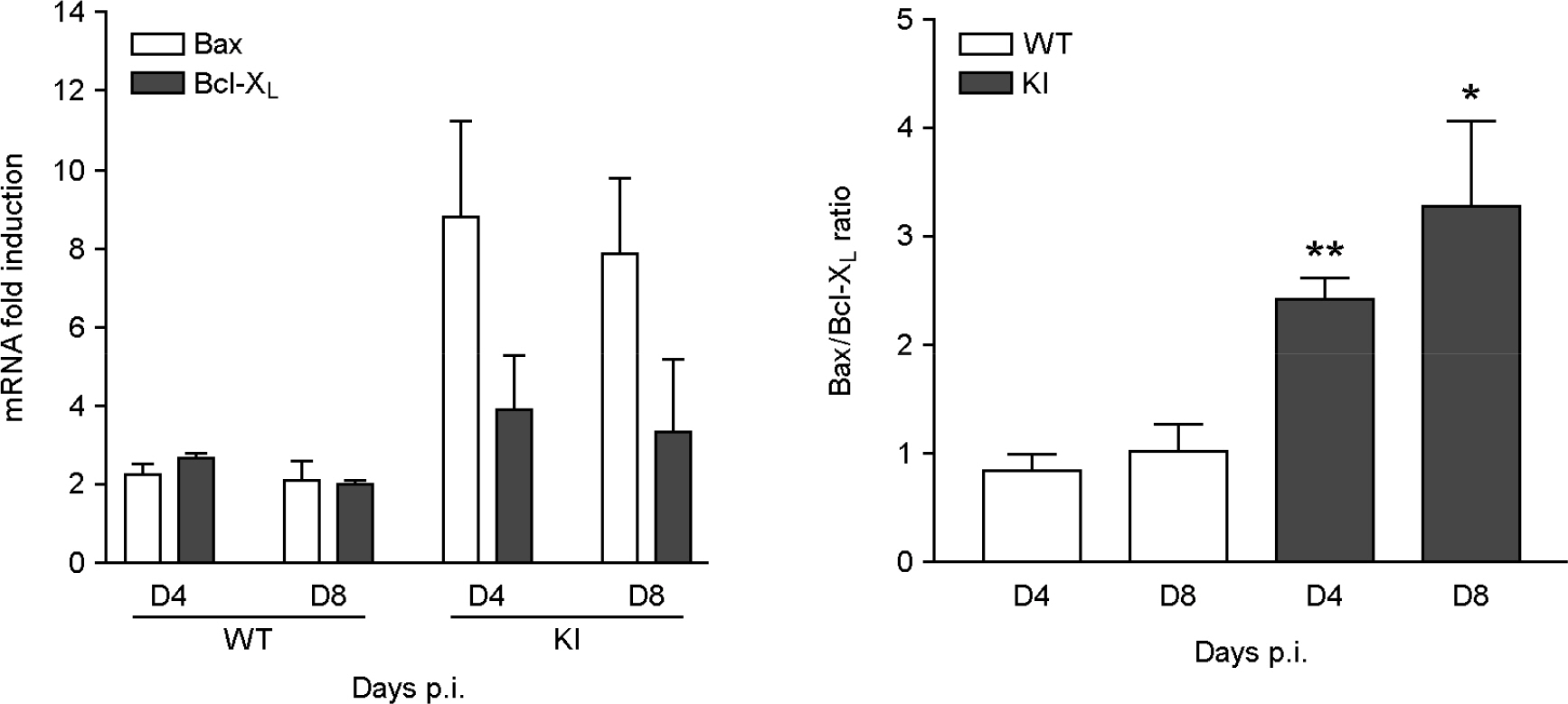
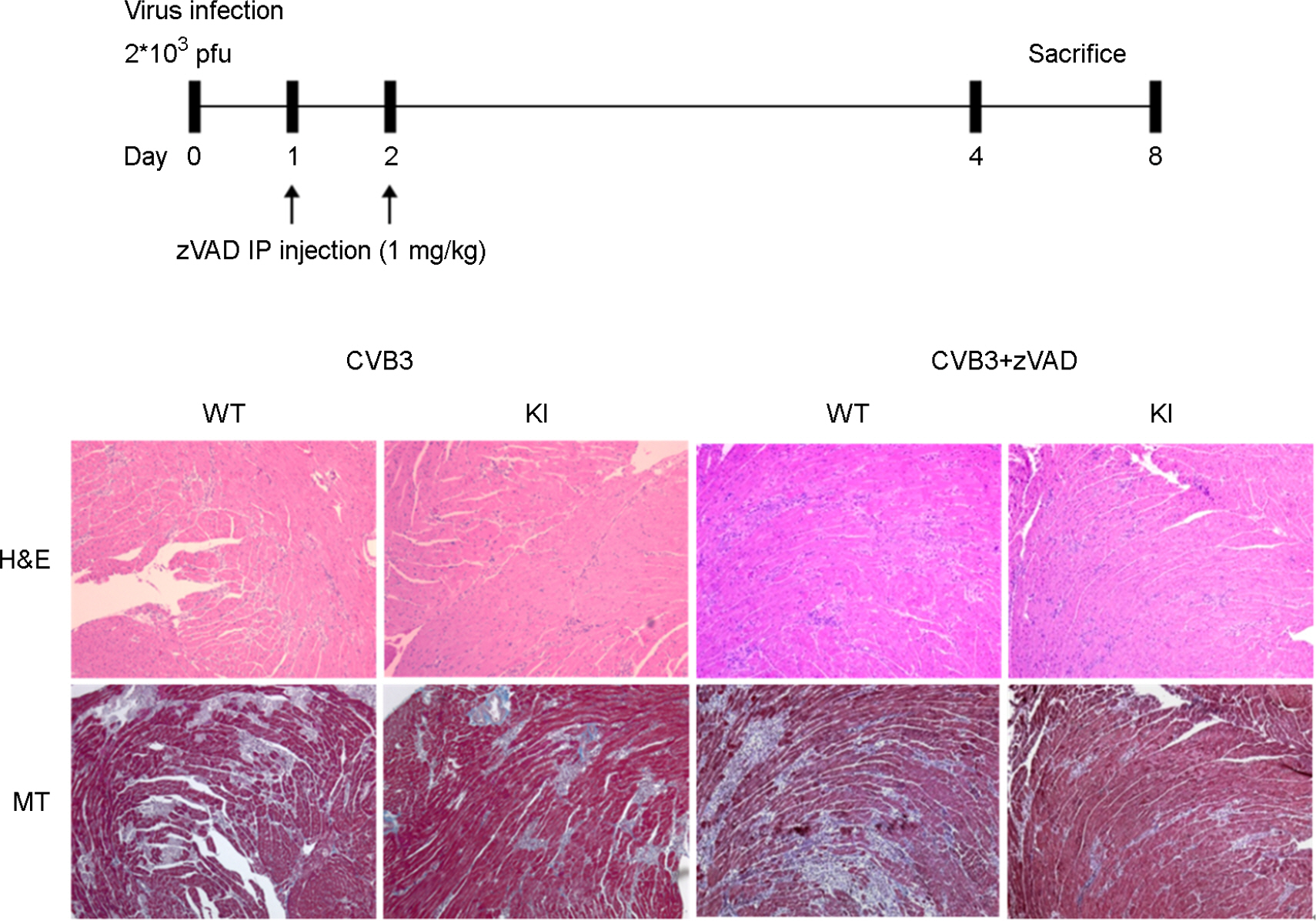
- It has been previously demonstrated that dystrophin is cleaved in the cardiac myocyte by the viral protease 2A following infection with Coxsackievirus B3 (CVB3). The viral protease 2A mediated cardiomyopathy can be prevented by inhibiting cleavage of dystrophin. However, it is less clear whether uncleaved dysdrophin have other heart protective effect in coxsackievirus infection. To address this, we generated a Balb/C background mouse that had a point mutation in dystrophin that prevents cleavage by protease 2A (KI). We show here that when mice expressing cleavage-resistant dystrophin were infected with CVB3, there was increased cardiac myocyte apoptosis. Bax and Bcl-X(L) mRNA ratio was significantly increased in KI mice heart compare to wild type mice heart. We found cleavage-resistant dystrophin induced the apoptosis related enzyme capspase-3 and caspase-8 activity. In addition, TUNEL stain was observed many TUNEL positive cardiac myocyte in KI mice heart compare to wild type mice heart (3.7% vs 0.3%). However, zVAD treatment for apoptosis blocking was significantly decreased myocardium damage and fibrosis in KI mice heart. These findings indicated that uncleaved dystrophin may have a critical role in cardiac myocyte viral propagation. Uncleaved dystrophin mutant induced cardiac myocyte apoptosis. It delayed coxsackievirus propagation in cardiac myocyte and could prevent cardiac myocyte death.
Keyword
MeSH Terms
Figure
Reference
-
1). Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996; 70:7811–8.
Article2). Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999; 5:320–6.
Article3). Gradi A, Imataka H, Svitkin YV, Rom E, Raught B, Morino S, et al. A novel functional human eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998; 18:334–42.
Article4). Lamphear BJ, Yan R, Yang F, Waters D, Liebig HD, Klump H, et al. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993; 268:19200–3.
Article5). Castelló A, Alvarez E, Carrasco L. Differential cleavage of eIF4GI and eIF4GII in mammalian cells. Effects on translation. J Biol Chem. 2006; 281:33206–16.6). Xiong D, Lee GH, Badorff C, Dorner A, Lee S, Wolf P, et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002; 8:872–7.
Article7). Mavrogeni S, Papavasiliou A, Spargias K, Constandoulakis P, Papadopoulos G, Karanasios E, et al. Myocardial inflammation in Duchenne Muscular Dystrophy as a precipitating factor for heart failure: a prospective study. BMC Neurol. 2010; 10:33.
Article8). Lim BK, Peter AK, Xiong D, Narezkina A, Yung A, Dalton ND, et al. Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J Clin Invest. 2013; 123:5146–51.
Article9). Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 1997; 223:31–52.
Article10). Colston JT, Chandrasekar B, Freeman GL. Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc Res. 1998; 38:158–68.
Article11). Peng T, Sadusky T, Li Y, Coulton GR, Zhang H, Archard LC. Altered expression of Bag-1 in Coxsackievirus B3 infected mouse heart. Cardiovasc Res. 2001; 50:46–55.
Article12). Kytö V, Lapatto R, Lakkisto P, Saraste A, Voipio-Pulkki LM, Vuorinen T, et al. Glutathione depletion and cardiomyocyte apoptosis in viral myocarditis. Eur J Clin Invest. 2004; 34:167–75.
Article13). Yun SH, Lee WG, Kim YC, Ju ES, Lim BK, Choi JO, et al. Antiviral activity of coxsackievirus B3 3C protease inhibitor in experimental murine myocarditis. J Infect Dis. 2012; 205:491–7.
Article14). Van Noorden CJ. The history of Z-VAD-FMK, a tool for understanding the significance of caspase inhibition. Acta Histochem. 2001; 103:241–51.
Article15). Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999; 13:1899–911.
Article16). Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995; 376:37–43.
Article17). Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999; 99:1091–100.18). Suzuki K, Kostin S, Person V, Elsässer A, Schaper J. Time course of the apoptotic cascade and effects of caspase inhibitors in adult rat ventricular cardiomyocytes. J Mol Cell Cardiol. 2001; 33:983–94.
Article19). Huber SA. T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res. 2000; 45:579–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac-specific Coxsackievirus and Adenovirus Receptor (CAR) Deletion Inhibit Enterovirus Infection in Murine Heart
- Cardiac Myocyte Cell Death in Isoproterenol-Induced Cardiac Hypertrophy in Rats
- Effects of iNOS inhibitor on IFN-gamma production and apoptosis of splenocytes in genetically different strains of mice infected with Toxoplasma gondii
- Development of a Gene Therapy Method for Cervical Cancer Using Attenuated Coxsackievirus B3 as a Vector System
- Expression of Plus- and Minus-strand Viral RNA in Coxsackievirus B3-Infected A/J Mice