J Vet Sci.
2009 Sep;10(3):211-218. 10.4142/jvs.2009.10.3.211.
Efficacy of strain RB51 vaccine in protecting infection and vertical transmission against Brucella abortus in Sprague-Dawley rats
- Affiliations
-
- 1Korean Zoonoses Research Institute, Chonbuk National University, Jeonju 561-756, Korea. baekbk@chonbuk.ac.kr
- 2Division of Model Animal, Institute of Biomedical Science, Kansai Medical University, Osaka, 570-8506, Japan.
- KMID: 1726920
- DOI: http://doi.org/10.4142/jvs.2009.10.3.211
Abstract
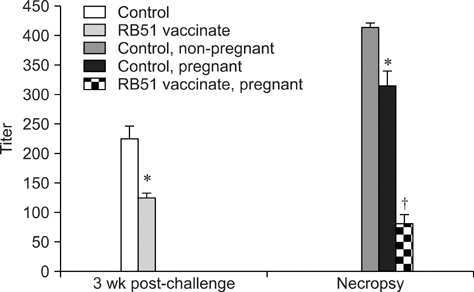
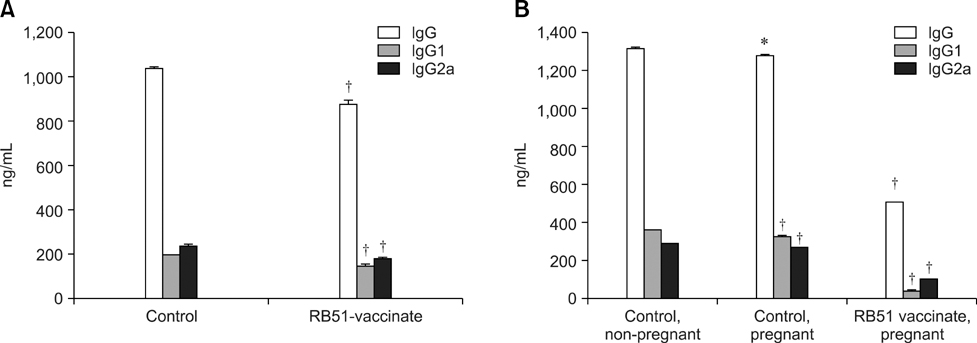
- Immunizing animals in the wild against Brucella (B.) abortus is essential to control bovine brucellosis because cattle can get the disease through close contact with infected wildlife. The aim of this experiment was to evaluate the effectiveness of the B. abortus strain RB51 vaccine in protecting infection as well as vertical transmission in Sprague-Dawley (SD) rats against B. abortus biotype 1. Virgin female SD rats (n = 48) two months of age were divided into two groups: one group (n = 24) received RB51 vaccine intraperitoneally with 3 x 10(10) colony forming units (CFU) and the other group (n = 24) was used as non-vaccinated control. Non-vaccinated and RB51-vaccinated rats were challenged with 1.5 x 10(9) CFU of virulent B. abortus biotype 1 six weeks after vaccination. Three weeks after challenge, all rats were bred. Verification of RB51-vaccine induced protection in SD rats was determined by bacteriological, serological and molecular screening of maternal and fetal tissues at necropsy. The RB51 vaccine elicited 81.25% protection in SD rats against infection with B. abortus biotype 1. Offspring from rats vaccinated with RB51 had a decreased (p < 0.05) prevalence of vertical transmission of B. abortus biotype 1 compared to the offspring from non-vaccinated rats (20.23% and 87.50%, respectively). This is the first report of RB51 vaccination efficacy against the vertical transmission of B. abortus in the SD rat model.
MeSH Terms
-
Animals
Antibodies, Bacterial/blood
Bacterial Vaccines/immunology/*standards
Birth Weight
Brucella abortus/immunology/isolation & purification/*physiology
Brucellosis/immunology/microbiology/*prevention & control/*transmission
Female
Infectious Disease Transmission, Vertical/*prevention & control
Litter Size
Male
Pregnancy
Pregnancy Rate
Rats
Rats, Sprague-Dawley
Survival Analysis
Figure
Reference
-
1. Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. 1988. Paris: Institut National de la Recherche Agronomique;17–136.2. Baek BK, Lee BO, Hur J, Rahman MS, Lee SI, Kakoma I. Evaluation of the Sprague-Dawley rat as a model for vertical transmission of Brucella abortus. Can J Vet Res. 2005. 69:305–308.3. Bosseray N. Mother to young transmission of Brucella abortus infection in mouse model. Ann Rech Vet. 1982. 13:341–349.4. Catlin JE, Sheehan EJ. Transmission of bovine brucellosis from dam to offspring. J Am Vet Med Assoc. 1986. 188:867–869.5. Cheville NF, Jensen AE, Halling SM, Tatum FM, Morfitt DC, Hennager SG, Frerichs WM, Schurig G. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am J Vet Res. 1992. 53:1881–1888.6. Cheville NF, Olsen SC, Jensen AE, Stevens MG, Palmer MV, Florance AM. Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am J Vet Res. 1996. 57:1153–1156.7. Cheville NF, Stevens MG, Jensen AE, Tatum FM, Halling SM. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993. 54:1591–1597.8. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997. 3:213–221.
Article9. Cutler SJ, Whatmore AM, Commander NJ. Brucellosis-new aspects of an old disease. J Appl Microbiol. 2005. 98:1270–1281.10. Davis DS, Elzer PH. Brucella vaccines in wildlife. Vet Microbiol. 2002. 90:533–544.11. Elzer PH, Enright FM, Colby L, Hagius SD, Walker JV, Fatemi MB, Kopec JD, Beal VC Jr, Schurig GG. Protection against infection and abortion induced by virulent challenge exposure after oral vaccination of cattle with Brucella abortus strain RB51. Am J Vet Res. 1998. 59:1575–1578.12. Godfroid J. Brucellosis in wildlife. Rev Sci Tech. 2002. 21:277–286.13. Jiang X, Baldwin CL. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993. 61:124–134.
Article14. Jiménez De Bagüés MP, Elzer PH, Jones SM, Blasco JM, Enright FM, Schurig GG, Winter AJ. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994. 62:4990–4996.
Article15. Lapraik RD, Moffat R. Latent bovine brucellosis. Vet Rec. 1982. 111:578–579.16. López-Goñi I, García-Yoldí D, Marin CM, de Miguel MJ, Muñoz PM, Blasco JM, Jacques I, Grayon M, Cloeckaert A, Ferreira AC, Cardoso R, Corrêa de Sá MI, Walravens K, Albert D, Garin-Bastuji B. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol. 2008. 46:3484–3487.
Article17. Moore CG, Schnurrenberger PR. A review of naturally occurring Brucella abortus infections in wild mammals. J Am Vet Med Assoc. 1981. 179:1105–1112.18. Moriyón I, Grilló MJ, Monreal D, González D, Marín C, López-Góñi I, Mainar-Jaime RC, Moreno E, Blasco JM. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004. 35:1–38.
Article19. Ol'iakova NV, Antoniuk VI. The gray rat (Rattus norvegicus) as a carrier of infectious causative agents in Siberia and the Far East. Med Parazitol (Mosk). 1989. May-Jun. 73–77.20. Olsen SC. Responses of adult cattle to vaccination with a reduced dose of Brucella abortus strain RB51. Res Vet Sci. 2000. 69:135–140.
Article21. Olsen SC, Jensen AE, Stoffregen WC, Palmer MV. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Res Vet Sci. 2003. 74:17–22.
Article22. Palmer MV, Olsen SC, Cheville NF. Safety and immunogenicity of Brucella abortus strain RB51 vaccine in pregnant cattle. Am J Vet Res. 1997. 58:472–477.23. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005. 352:2325–2336.
Article24. Park MY, Lee CS, Choi YS, Park SJ, Lee JS, Lee HB. A sporadic outbreak of human brucellosis in Korea. J Korean Med Sci. 2005. 20:941–946.
Article25. Pasquali P, Rosanna A, Pistoia C, Petrucci P, Ciuchini F. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect Immun. 2003. 71:2326–2330.
Article26. Poester FP, Gonçalves VSP, Paixão TA, Santos RL, Olsen SC, Schurig GG, Lage AP. Efficacy of strain RB51 vaccine in heifers against experimental brucellosis. Vaccine. 2006. 24:5327–5334.
Article27. Rhyan JC, Quinn WJ, Stackhouse LS, Henderson JJ, Ewalt DR, Payeur JB, Johnson M, Meagher M. Abortion caused by Brucella abortus biovar 1 in a free-ranging bison (Bison bison) from Yellowstone National Park. J Wildl Dis. 1994. 30:445–446.
Article28. Schurig GG, Roop RM II, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991. 28:171–188.
Article29. Stevens MG, Hennager SG, Olsen SC, Cheville NF. Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J Clin Microbiol. 1994. 32:1065–1066.
Article30. Stevens MG, Olsen SC. Antibody responses to Brucella abortus 2308 in Cattle vaccinated with B. abortus RB51. Infect Immun. 1996. 64:1030–1034.
Article31. Stevens MG, Olsen SC, Cheville NF. Comparative analysis of immune responses in cattle vaccinated with Brucella abortus strain 19 or strain RB51. Vet Immunol Immunopathol. 1995. 44:223–235.
Article32. Stevens MG, Olsen SC, Pugh GW Jr, Brees D. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella abortus 19 or RB51. Infect Immun. 1995. 63:264–270.
Article33. Wee SH, Nam HM, Kim CH. Emergence of brucellosis in cattle in the Republic of Korea. Vet Rec. 2008. 162:556–557.
Article34. Wilesmith JW. The persistence of Brucella abortus infection in calves: a retrospective study of heavily infected herds. Vet Rec. 1978. 103:149–153.
Article35. Yaeger M, Holler LD. Youngquist RS, editor. Bacterial causes of bovine infertility and abortion. Current Therapy in Large Animal Theriogenology. 1997. Philadelphia: Saunders;364–372.
Article36. Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995. 21:283–289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Verification of immunosuppression in chicks caused by Cryptosporidium baileyi infection using Brucella abortus strain 1119-3
- Vaccination of goats with a combination Salmonella vector expressing four Brucella antigens (BLS, PrpA, Omp19, and SOD) confers protection against Brucella abortus infection
- Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice
- Development and trial of vaccines against Brucella
- Diversity of Humoral Immune Responses to Recombinant Proteins of Brucella abortus Among Residents in Cheju Province