J Vet Sci.
2009 Sep;10(3):197-201. 10.4142/jvs.2009.10.3.197.
Role of protease inhibitors and acylation stimulating protein in the adipogenesis in 3T3-L1 cells
- Affiliations
-
- 1Department of Biochemistry, Faculty of Veterinary Medicine, Benha University, 020-013, Egypt. mohamedsoliman8896@yahoo.com
- 2Department of Histology, Faculty of Medicine, Benha University, 020-013, Egypt.
- 3Department of Biomedical Sciences, Laboratory of Biochemistry, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, 060-0818, Japan.
- KMID: 1726918
- DOI: http://doi.org/10.4142/jvs.2009.10.3.197
Abstract
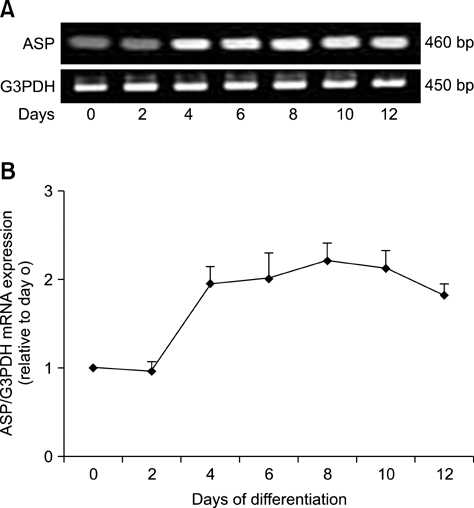
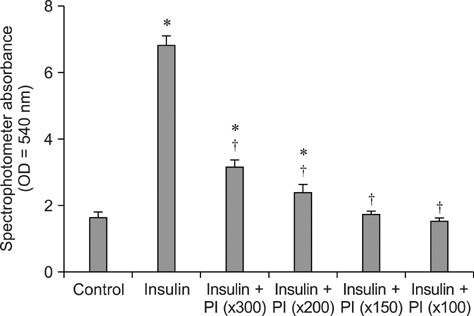
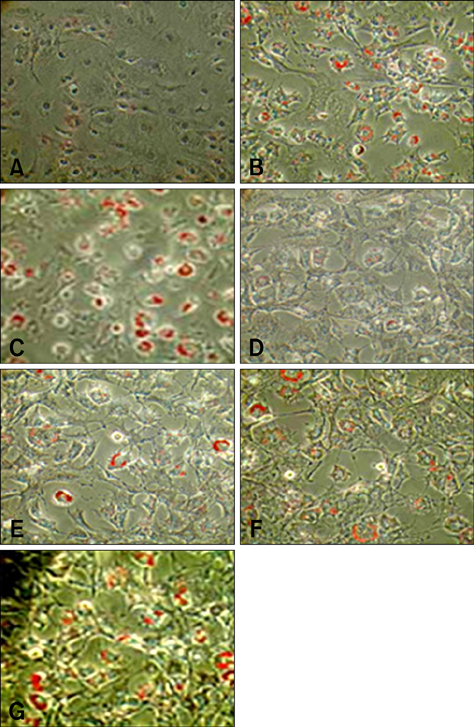
- Treatment of AIDS (HIV) and hepatitis C virus needs protease inhibitors (PI) to prevent viral replication. Uses of PI in therapy are usually associated with a decrease in body weight and dyslipidemia. Acylation stimulating protein (ASP) is a protein synthesized in adipocytes to increase triglycerides biosynthesis, for that the relation of PI and ASP to adipogenesis is tested in this work. ASP expression was increased during 3T3-L1 differentiation and reached a peak at day 8 with cell maturation. Addition of PI during adipocytes differentiation dose dependently and significantly (p < 0.5) inhibited the degree of triglycerides (TG) accumulation. Moreover, presence of ASP (450 ng/mL) in media significantly (p < 0.5) stimulated the degree of TG accumulation and there was additive stimulation for ASP when added with insulin (10 microgram/mL). Finally, when ASP in different doses (Low, 16.7; Medium, 45 and High, 450 ng/mL) incubated with a dose of x150 PI, ASP partially inhibited the PI-inhibited adipogenesis and TG accumulation. The results in this study show that PI inhibit lipids accumulation and confirm role of ASP in TG biosynthesis and adipogenesis.
MeSH Terms
Figure
Reference
-
1. Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, Zhou G, Biswas C, Cullinan CA, Hayes NS, Li Y, Tanen M, Ventre J, Wu MS, Berger GD, Mosley R, Marquis R, Santini C, Sahoo SP, Tolman RL, Smith RG, Moller DE. Novel peroxisome proliferator-activated receptor (PPAR) γ and PPARδ ligands produce distinct biological effects. J Bio Chem. 1999. 274:6718–6725.
Article2. Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001. 50:1378–1388.
Article3. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998. 12:F51–F58.
Article4. Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998. 351:1881–1883.
Article5. Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992. 267:12736–12741.
Article6. Cianflone K, Maslowska M. Differentiation-induced production of ASP in human adipocytes. Eur J Clin Invest. 1995. 25:817–825.
Article7. Cianflone KM, Sniderman AD, Walsh MJ, Vu HT, Gagnon J, Rodriguez MA. Purification and characterization of acylation stimulating protein. J Biol Chem. 1989. 264:426–430.
Article8. Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987. 237:402–404.
Article9. De Barros S, Zakaroff-Girard A, Lafontan M, Galitzky J, Bourlier V. Inhibition of human preadipocyte proteasomal activity by HIV Protease Inhibitors or specific inhibitor lactacystin leads to a defect in adipogenesis, which involves matrix metalloproteinase-9. J Pharmacol Exp Ther. 2007. 320:291–299.
Article10. Gartner LP, Hiatt JL. Color Textbook of Histology. 2007. 3rd ed. Philadelphia: Saunders;114–123.11. Germinario R, Sniderman AD, Manuel S, Lefebvre SP, Baldo A, Cianflone K. Coordinate regulation of triacylglycerol synthesis and glucose transport by acylation-stimulating protein. Metabolism. 1993. 42:574–580.
Article12. Grant A, Helferich G. Pearson AM, Dutson TR, editors. An overeview of growth. Growth Regulation in Farm Animals. 1991. London: Elsevier Applied Science;1–16.13. Jacobi SK, Miner JL. Human acylation-stimulating protein and lipid biosynthesis in bovine adipose tissue explants. J Anim Sci. 2002. 80:751–756.
Article14. Junqueira LC, Carneiro J. Basic Histology Text & Atlas. 2005. 11th ed. New York: McGraw-Hill;123–127.15. Koistinen HA, Vidal H, Karonen SL, Dusserre E, Vallier P, Koivisto VA, Ebeling P. Plasma acylation stimulating protein concentration and subcutaneous adipose tissue C3 mRNA expression in nondiabetic and type 2 diabetic men. Arterioscler Thromb Vasc Biol. 2001. 21:1034–1039.
Article16. Maslowska M, Legakis H, Assadi F, Cianflone K. Targeting the signaling pathway of acylation stimulating protein. J Lipid Res. 2006. 47:643–652.
Article17. Moller DE, O'Rahilly S. Mollere DE, editor. Syndromes of severe insulin resistance: clinical and pathophysiological features. Insulin Resistance. 1993. New York: John Wiley & Sons;49–82.18. Muscari A, Bozzoli C, Puddu GM, Rovinetti C, Fiorentini GP, Roversi RA, Puddu P. Correlations between serum lipids and complement components in adults without demonstrated atherosclerotic disease. Atherosclerosis. 1990. 81:111–118.
Article19. Ross MH, Pawlina W. Histology: A Text and Atlas. 2006. 5th ed. London: Lippncott Williams & Wilkins;238–246.20. Tahiri Y, Karpe F, Tan GD, Cianflone K. Rosiglitazone decreases postprandial production of acylation stimulating protein in type 2 diabetics. Nutr Metab (Lond). 2007. 4:11.
Article21. Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. 1995. 5:571–576.
Article22. Vigouroux C, Gharakhanian S, Salhi Y, Nguyen TH, Chevenne D, Capeau J, Rozenbaum W. Diabetes, insulin resistance and dyslipidaemia in lipodystrophic HIV-infected patients on highly active antiretroviral therapy (HAART). Diabetes Metab. 1999. 25:225–232.23. Walli R, Herfort O, Michl GM, Demant T, Jäger H, Dieterle C, Bogner JR, Landgraf R, Goebel FD. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998. 12:F167–F173.
Article24. Xia Z, Stanhope KL, Digitale E, Simion OM, Chen L, Havel P, Cianflone K. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. J Biol Chem. 2004. 279:4051–4057.
Article25. Zhang B, Macnaul K, Szalkowski D, Li Z, Berger J, Moller DE. Inhibition of adipocyte differentiation by HIV protease inhibitors. J Clin Endocrinol Metab. 1999. 84:4274–4277.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Cydonia oblonga Miller fruit extract exerts an anti-obesity effect in 3T3-L1 adipocytes by activating the AMPK signaling pathway