J Vet Sci.
2009 Sep;10(3):181-187. 10.4142/jvs.2009.10.3.181.
Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- Affiliations
-
- 1Adult Stem Cell Research Center, College of Veterinery Medicine, Seoul National University, Seoul 151-742, Korea.
- 2Laboratory of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinery Medicine, Seoul National University, Seoul 151-742, Korea.
- 3BK 21 program for Veterinary Sciences, College of Veterinery Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 1726916
- DOI: http://doi.org/10.4142/jvs.2009.10.3.181
Abstract
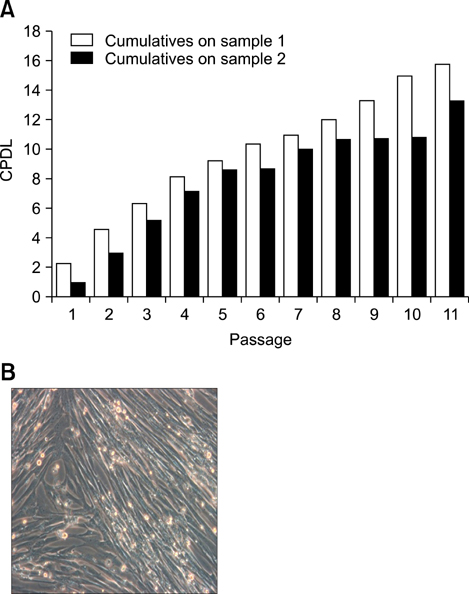
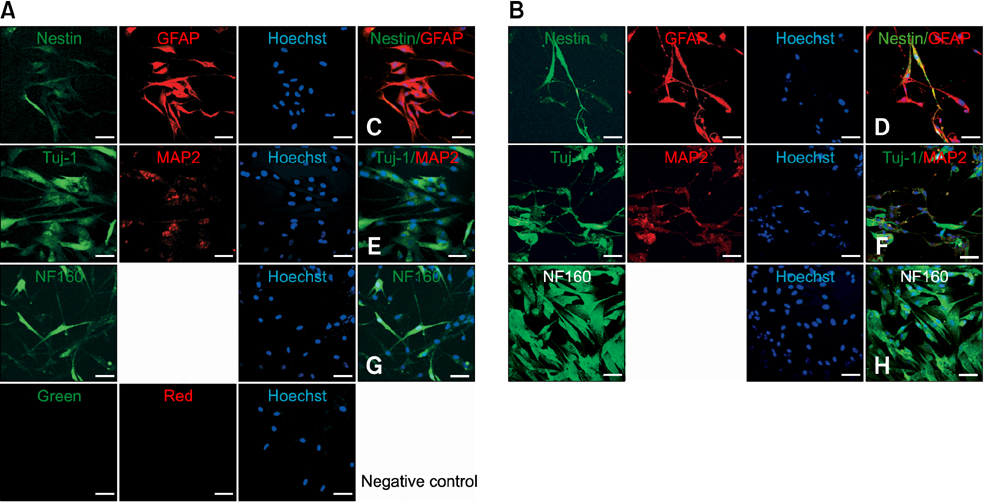
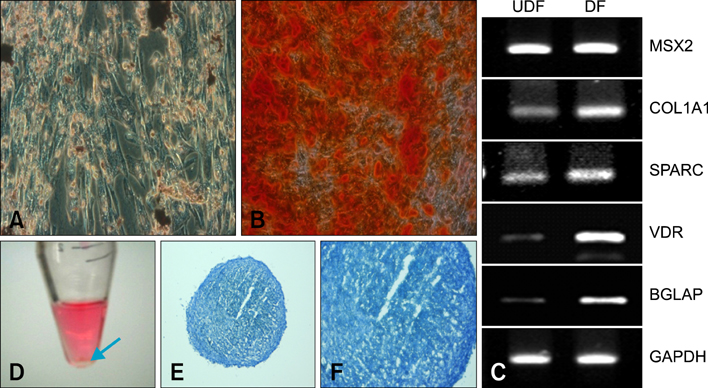
- Human umbilical cord blood-derived mesenchymal stem cells (MSCs) are known to possess the potential for multiple differentiations abilities in vitro and in vivo. In canine system, studying stem cell therapy is important, but so far, stem cells from canine were not identified and characterized. In this study, we successfully isolated and characterized MSCs from the canine umbilical cord and its fetal blood. Canine MSCs (cMSCs) were grown in medium containing low glucose DMEM with 20% FBS. The cMSCs have stem cells expression patterns which are concerned with MSCs surface markers by fluorescence-activated cell sorter analysis. The cMSCs had multipotent abilities. In the neuronal differentiation study, the cMSCs expressed the neuronal markers glial fibrillary acidic protein (GFAP), neuronal class III beta tubulin (Tuj-1), neurofilament M (NF160) in the basal culture media. After neuronal differentiation, the cMSCs expressed the neuronal markers Nestin, GFAP, Tuj-1, microtubule-associated protein 2, NF160. In the osteogenic & chondrogenic differentiation studies, cMSCs were stained with alizarin red and toluidine blue staining, respectively. With osteogenic differentiation, the cMSCs presented osteoblastic differentiation genes by RT-PCR. This finding also suggests that cMSCs might have the ability to differentiate multipotentially. It was concluded that isolated MSCs from canine cord blood have multipotential differentiation abilities. Therefore, it is suggested that cMSCs may represent a be a good model system for stem cell biology and could be useful as a therapeutic modality for canine incurable or intractable diseases, including spinal cord injuries in future regenerative medicine studies.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix
Talita F.B. Souza, Silmara S. Sakamoto, Gabriel T.N.M. Ferreira, Roberto Gameiro, Marcia Marinho, Alexandre L. de Andrade, Tereza C. Cardoso
J Vet Sci. 2015;16(3):381-384. doi: 10.4142/jvs.2015.16.3.381.
Reference
-
1. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.
Article2. Bernard BA. Human skin stem cells. J Soc Biol. 2008. 202:3–6.3. Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000. 95:581–585.
Article4. Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004. 22:625–634.
Article5. Breems DA, Löwenberg B. Acute myeloid leukemia and the position of autologous stem cell transplantation. Semin Hematol. 2007. 44:259–266.
Article6. Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci USA. 1998. 95:10614–10619.
Article7. Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006. 24:1054–1064.
Article8. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–3843.
Article9. Donahue RE, Kuramoto K, Dunbar CE. Large animal models for stem and progenitor cell analysis. Curr Protoc Immunol. 2005. Chapter 22:Unit 22A. 1.
Article10. Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2004. 321:102–108.
Article11. Guo JS, Zeng YS, Li HB, Huang WL, Liu RY, Li XB, Ding Y, Wu LZ, Cai DZ. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord. 2007. 45:15–24.
Article12. Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008. 4:3–11.
Article13. Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W, Baan CC. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007. 16:597–604.
Article14. Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004. 6:543–553.
Article15. Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 2006. 20:950–952.
Article16. Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004. 6:532–539.
Article17. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
Article18. Jordan PM, Ojeda LD, Thonhoff JR, Gao J, Boehning D, Yu Y, Wu P. Generation of spinal motor neurons from human fetal brain-derived neural stem cells: role of basic fibroblast growth factor. J Neurosci Res. 2009. 87:318–332.
Article19. Jurga M, Markiewicz I, Sarnowska A, Habich A, Kozlowska H, Lukomska B, Buzanska L, Domanska-Janik K. Neurogenic potential of human umbilical cord blood: neural-like stem cells depend on previous long-term culture conditions. J Neurosci Res. 2006. 83:627–637.
Article20. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.
Article21. Kim JW, Kim SY, Park SY, Kim YM, Kim JM, Lee MH, Ryu HM. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004. 83:733–738.
Article22. Krampera M, Marconi S, Pasini A, Galiè M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007. 40:382–390.
Article23. Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990. 40:11–15.24. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004. 103:1669–1675.
Article25. Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005. 11:787–802.
Article26. Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006. 117:725–738.
Article27. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999. 48:913–927.
Article28. Orino K, Uehara M, Okano S, Watanabe K. Purification and characterization of canine serum ferritin-binding proteins. Biometals. 2006. 19:315–322.
Article29. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article30. Storb R, Yu C, Deeg HJ, Georges G, Kiem HP, McSweeney PA, Nash RA, Sandmaier BM, Sullivan KM, Wagner JL, Walters MC. Current and future preparative regimens for bone marrow transplantation in thalassemia. Ann N Y Acad Sci. 1998. 850:276–287.
Article31. Suter SE, Gouthro TA, McSweeney PA, Nash RA, Haskins ME, Felsburg PJ, Henthorn PS. Isolation and characterization of pediatric canine bone marrow CD34+ cells. Vet Immunol Immunopathol. 2004. 101:31–47.
Article32. Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002. 12:502–508.
Article33. Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am J Vet Res. 2005. 66:1729–1737.
Article34. Wang JF, Wang LJ, Wu YF, Xiang Y, Xie CG, Jia BB, Harrington J, McNiece IK. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004. 89:837–844.35. Wang X, Li F, Niyibizi C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: implications of cell therapy for skeletal diseases. Stem Cells. 2006. 24:1867–1878.36. Wei Y, Hu Y, Lv R, Li D. Regulation of adipose-derived adult stem cells differentiating into chondrocytes with the use of rhBMP-2. Cytotherapy. 2006. 8:570–579.
Article37. Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005. 23:3–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Stem cell properties of cells derived from canine periodontal ligament
- In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood