J Vet Sci.
2009 Dec;10(4):331-336. 10.4142/jvs.2009.10.4.331.
Agar gel immunodiffusion analysis using baculovirus-expressed recombinant bovine leukemia virus envelope glycoprotein (gp51/gp30T-)
- Affiliations
-
- 1National Veterinary Research and Quarantine Service, Anyang 430-757, Korea. kweonch@mail.nvrqs.go.kr
- KMID: 1726906
- DOI: http://doi.org/10.4142/jvs.2009.10.4.331
Abstract
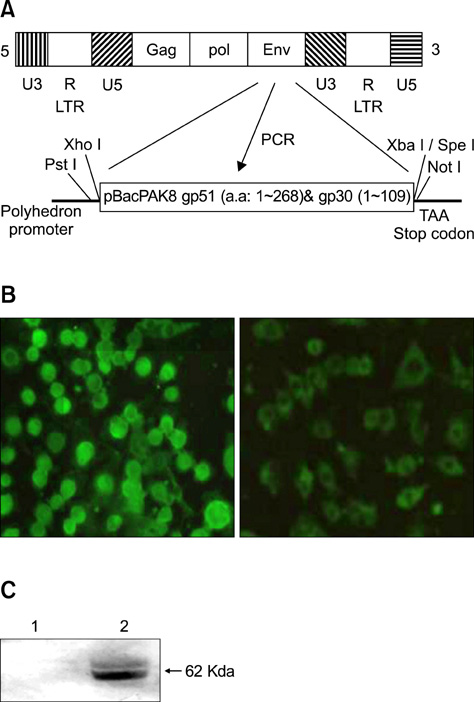
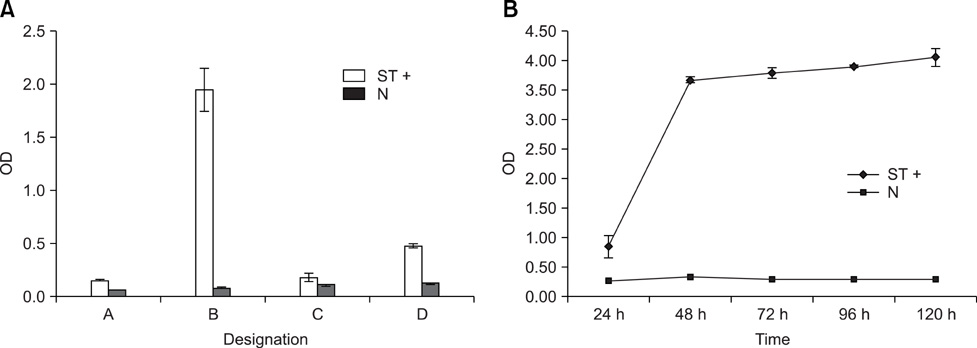
- Bovine leukemia virus (BLV) envelope glycoprotein (gp51/gp30T-), consisting of BLV gp51 and BLV gp30 that lacked its C-terminal transmembrane domain, was expressed in insect cells under the control of the baculovirus polyhedron promoter. Recombinant BLV gp51/gp30T- secreted from insect cells was determined by immunofluorescence, enzyme-linked immunosorbent and western blot assays using a BLV-specific monoclonal antibody and BLV-positive bovine antibodies. An agar gel immunodiffusion (AGID) test using gp51/gp30T- as the antigen for the detection of BLV antibodies in serum was developed and compared to traditional AGID, which uses wild type BLV antigen derived from fetal lamb kidney cells. AGID with the recombinant BLV gp51/gp30T- was relatively more sensitive than traditional AGID. When the two methods were tested with bovine sera from the field, the recombinant BLV gp51/gp30T- and traditional antigen had a relative sensitivity of 69.8% and 67.4%, respectively, and a relative specificity of 93.3% and 92.3%. These results indicated that the recombinant BLV gp51/gp30T- is an effective alternative antigen for the diagnosis of BLV infection in cattle.
MeSH Terms
-
Agar
Animals
Antibodies, Viral/blood
Antigens, Viral/immunology
Baculoviridae/*metabolism
Cattle
Cell Line
Enzootic Bovine Leukosis/blood/immunology
Gene Expression Regulation, Viral/*physiology
Immunodiffusion/methods/*veterinary
Kidney/cytology
Leukemia Virus, Bovine/genetics/*metabolism
Molecular Biology
Sheep
Viral Envelope Proteins/genetics/*metabolism
Figure
Reference
-
1. Ballagi-Pordány A, Klintevall K, Merza M, Klingeborn B, Belák S. Direct detection of bovine leukemia virus infection: practical applicability of a double polymerase chain reaction. Zentralbl Veterinarmed B. 1992. 39:69–77.
Article2. Beier D, Riebe R, Blankenstein P, Starick E, Bondzio A, Marquardt O. Establishment of a new bovine leukosis virus producing cell line. J Virol Methods. 2004. 121:239–246.
Article3. Callebaut I, Vonèche V, Mager A, Fumière O, Krchnak V, Merza M, Zavada J, Mammerickx M, Burny A, Portetelle D. Mapping of B-neutralizing and T-helper cell epitopes on the bovine leukemia virus external glycoprotein gp51. J Virol. 1993. 67:5321–5327.
Article4. Chi J, VanLeeuwen JA, Weersink A, Keefe GP. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev Vet Med. 2002. 55:137–153.
Article5. Choi KY, Liu RB, Buehring GC. Relative sensitivity and specificity of agar gel immunodiffusion, enzyme immunosorbent assay, and immunoblotting for detection of anti-bovine leukemia virus antibodies in cattle. J Virol Methods. 2002. 104:33–39.
Article6. De Giuseppe A, Feliziani F, Rutili D, De Mia GM. Expression of the bovine leukemia virus envelope glycoprotein (gp51) by recombinant baculovirus and its use in an enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2004. 11:147–151.
Article7. Doménech A, Llames L, Goyache J, Suárez G, Gómez-Lucía E. Macrophages infected with bovine leukaemia virus (BLV) induce humoral response in rabbits. Vet Immunol Immunopathol. 1997. 58:309–320.
Article8. Gatei MH, Good MF, Daniel RC, Lavin MF. T-cell responses to highly conserved CD4 and CD8 epitopes on the outer membrane protein of bovine leukemia virus: Relevance to vaccine development. J Virol. 1993. 67:1796–1802.
Article9. Johnson M, Rommel F, Moné J. Development of a syncytia inhibition assay for the detection of antibodies to bovine leukemia virus in naturally infected cattle; comparison with Western blot and agar gel immunodiffusion. J Virol Methods. 1998. 70:177–182.
Article10. Johnston ER, Radke K. The SU and TM envelope protein subunits of Bovine leukemia virus are linked by disulfide bonds, both in cells and in virions. J Virol. 2000. 74:2930–2935.
Article11. Kittelberger R, Reichel MP, Meynell RM, Tham KM, Molloy JB. Detection of antibodies against the core protein p24 of the bovine leukemia virus in cattle for confirmatory serological testing. J Virol Methods. 1999. 77:109–114.
Article12. Kuckleburg CJ, Chase CC, Nelson EA, Marras SA, Dammen MA, Christopher-Hennings J. Detection of bovine leukemia virus in blood and milk by nested and real-time polymerase chain reactions. J Vet Diagn Invest. 2003. 15:72–76.
Article13. Kweon CH, Ko YJ, Kim WI, Lee SY, Nah JJ, Lee KN, Sohn HJ, Choi KS, Hyun BH, Kang SW, Joo YS, Lubroth J. Development of a foot-and-mouth disease NSP ELISA and its comparison with differential diagnostic methods. Vaccine. 2003. 21:1409–1414.
Article14. Kweon CH, Kwon BJ, Kim IJ, Lee SY, Ko YJ. Development of monoclonal antibody-linked ELISA for sero-diagnosis of vesicular stomatitis virus (VSV-IN) using baculovirus expressed glycoprotein. J Virol Methods. 2005. 130:7–14.
Article15. Kweon CH, Kwon BJ, Ko YJ, Kenichi S. Development of competitive ELISA for serodiagnosis on African horsesickness virus using baculovirus expressed VP7 and monoclonal antibody. J Virol Methods. 2003. 113:13–18.
Article16. Miller JM, Van Der Maaten MJ. Use of glycoprotein antigen in the immunodiffusion test for bovine leukemia virus antibodies. Eur J Cancer. 1977. 13:1369–1375.
Article17. Office International des Epizooties (OIE). Chapter 2.3.4. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2004. Paris: OIE.18. Portetelle D, Dandoy C, Burny A, Zavada J, Siakkou H, Gras-Masse H, Drobecq H, Tartar A. Synthetic peptides approach to identification of epitopes on bovine leukemia virus envelope glycoprotein gp51. Virology. 1989. 169:34–41.
Article19. Portetelle D, Mammerickx M, Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukaemia virus envelope protein gp51. J Virol Methods. 1989. 23:211–222.
Article20. Reichert M, Winnicka A, Willems L, Kettmann R, Cantor GH. Role of the proline-rich motif of bovine leukemia virus transmembrane protein gp30 in viral load and pathogenicity in sheep. J Virol. 2001. 75:8082–8089.
Article21. Russo S, Montermini L, Berkovitz-Siman-Tov R, Ponti W, Poli G. Expression of bovine leukemia virus ENV glycoprotein in insect cells by recombinant baculovirus. FEBS Lett. 1998. 436:11–16.
Article22. Simard C, Richardson S, Dixon P, Komal J. Agar gel immunodiffusion test for the detection of bovine leukemia virus antibodies: lack of trans-Atlantic standardization. Can J Vet Res. 2000. 64:96–100.23. Trono KG, Pérez-Filgueira DM, Duffy S, Borca MV, Carrillo C. Seroprevalence of bovine leukemia virus in dairy cattle in Argentina: comparison of sensitivity and specificity of different detection methods. Vet Microbiol. 2001. 83:235–248.
Article24. Usui T, Meas S, Konnai S, Ohashi K, Onuma M. Seroprevalence of bovine immunodeficiency virus and bovine leukemia virus in dairy and beef cattle in Hokkaido. J Vet Med Sci. 2003. 65:287–289.
Article25. Van den Heuvel M, Portetelle D, Jefferson B, Jacobs RM. Adaptation of a sandwich enzyme-linked immunosorbent assay to determine the concentration of bovine leukemia virus p24 and optimal conditions for p24 expression in short-term cultures of peripheral blood mononuclear cells. J Virol Methods. 2003. 111:61–67.
Article26. Van der Maaten MJ, Miller JM, Schmerr MJ. In utero transmission of bovine leukemia virus. Am J Vet Res. 1981. 42:1052–1054.27. Van der Maaten MJ, Miller JM, Schmerr MJ. Effect of colostral antibody on bovine leukemia virus infection of neonatal calves. Am J Vet Res. 1981. 42:1498–1500.28. Willems L, Gatot JS, Mammerickx M, Portetelle D, Burny A, Kerkhofs P, Kettmann R. The YXXL signalling motifs of the bovine leukemia virus transmembrane protein are required for in vivo infection and maintenance of high viral loads. J Virol. 1995. 69:4137–4141.
Article29. Yokoyama WM. Production of monoclonal antibodies. Curr Protoc Cytom. 2006. Appendix 3. Appendix 3J.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Antibodies to Infectious Bursal Disease Virus (IBDV) by Agar Gel Immunodiffusion using Recombinant VP2 Protein
- Expression and Characterization of Human Immunodeficiency Virus Type 1 Envelope Mutant Glycoproteins by Using Baculovirus Expression System
- Development and evaluation of an immunochromatographic assay using a gp51 monoclonal antibody for the detection of antibodies against the bovine leukemia virus
- Cloning and Expression of Hemagglutinin-Neuraminidase Gene of a Thermostable Isolate of Newcastle Disease Virus by Baculovirus Recombinants
- Expression and Evaluation of Chikungunya Virus E1 and E2 Envelope Proteins for Serodiagnosis of Chikungunya Virus Infection