J Vet Sci.
2008 Sep;9(3):273-279. 10.4142/jvs.2008.9.3.273.
Evaluation of the effect of a 3rd GnRH injection administered six days after the 2nd GnRH injection of Ovsynch on the reproductive performance of Japanese black cows
- Affiliations
-
- 1The United Graduate School of Veterinary Science, Yamaguchi University, Yamaguchi 753-8515, Japan.
- 2Laboratory of Theriogenology, Kagoshima University, Kagoshima 890-0065, Japan. chikara@agri.kagoshima-u.ac.jp
- KMID: 1718580
- DOI: http://doi.org/10.4142/jvs.2008.9.3.273
Abstract
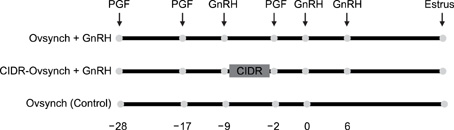
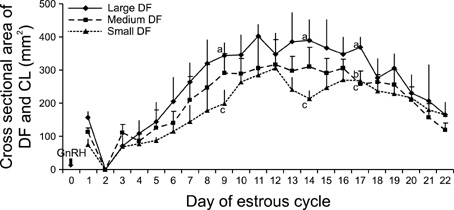
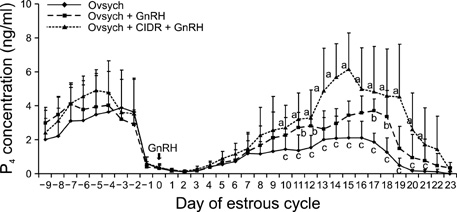
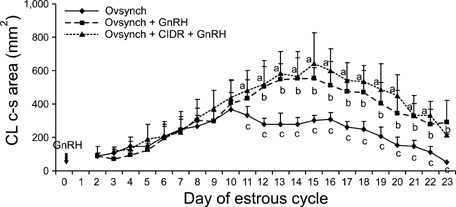
- This study was designed to evaluate the reproductive performance of Japanese black cows following the 3rd injection of gonadotropin releasing hormone (GnRH) analogue administered concurrently with Ovsynch-based treatment on day 6 (day 1 = the day of ovulation). In Experiment 1, 12 cows were allocated into three groups: a control group that was subjected to Ovsynch treatment and then injected with a placebo on day 6; group 1 (Ovsynch + GnRH), which was subjected to Ovsynch treatment and was injected with GnRH analogue on day 6, and group 2 (Ovsynch + controlled internal drug-release (CIDR) + GnRH), which received Ovsynch-CIDR treatment and was injected with GnRH analogue on day 6. Blood collection and ultrasonographic observation of the ovaries were conducted daily. Both treatments induced the formation of an accessory corpus luteum and significantly increased the cross-sectional area of the luteal tissue when compared to the control. However, plasma progesterone (P(4)) was significantly higher in the treatment groups than in the control group on days 11, 12, 17 and 18 in the group 1 and from day 10 to 21 in the group 2. In Experiment 2, 41 cows were assigned to the same three groups described above and then artificially inseminated on day 1. The pregnancy rates on day 45 did not differ among groups. In conclusion, administration of GnRH analogue on day 6 following Ovsynch-based treatment did not improve the reproductive performance of Japanese black cows, even though the P(4) concentration was higher in groups that received the GnRH.
Keyword
MeSH Terms
-
Animals
Cattle
Corpus Luteum/anatomy & histology/drug effects/physiology
Delayed-Action Preparations
Drug Administration Schedule
Estrus/drug effects/physiology
Female
Gonadotropin-Releasing Hormone/administration & dosage/*pharmacology
Japan
Ovulation/drug effects/physiology
Placebos
Progesterone/blood
Reproduction/drug effects/*physiology
Figure
Reference
-
1. Ahmad N, Schrick FN, Butcher RL, Inskeep EK. Effect of persistent follicles on early embryonic losses in beef cows. Biol Reprod. 1995. 52:1129–1135.2. Bartolome JA, Melendez P, Kelbert D, Swift K, McHale J, Hernandez J, Silvestre F, Risco CA, Arteche ACM, Thatcher WW, Archbald LF. Strategic use of Gonadotrophin-releasing hormone (GnRH) to increase pregnancy rate and reduce pregnancy loss in lactating dairy cows subjected to synchronization of ovulation and timed insemination. Theriogenology. 2005. 63:1026–1037.
Article3. Campanile G, Di Palo R, Neglia G, Vecchio D, Gasparrini B, Prandi A, Galiero G, D'Occhio MJ. Corpus luteum function and embryonic mortality in buffaloes treated with a GnRH agonist, hCG and progesterone. Theriogenology. 2007. 67:1393–1398.
Article4. Chenault JR, Kratzer DD, Rzepkowski RA, Goodwin MC. LH and FSH response of Holstein heifers to fertirelin acetate, gonadorelin and buserelin. Theriogenology. 1990. 34:81–98.
Article5. Dhali A, Mishra DP, Mech A, Karunakaran M, Rajkhowa C. Role of LH and prostaglandin F2α on the development and regression of corpus luteum in mithun (Bos frontalis) estrous cycle. Gen Comp Endocrinol. 2006. 149:173–181.
Article6. Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 1989. 72:68–78.
Article7. El-Zarkouny SZ, Cartmill JA, Hensley BA, Stevenson JS. Pregnancy in dairy cows after synchronized ovulation regimens with or without presynchronization and progesterone. J Dairy Sci. 2004. 87:1024–1037.
Article8. Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol. 2003. 1:88–96.9. Fuller GB, Hansel W. Regression of sheep corpora lutea after treatment with antibovine luteinizing hormone. J Anim Sci. 1970. 31:99–103.
Article10. Geisert RD, Morgan GL, Short EC Jr, Zavy MT. Endocrine events associated with endometrial function and conceptus development in cattle. Reprod Fertil Dev. 1992. 4:301–305.
Article11. Howard JM, Manzo R, Dalton JC, Frago F, Ahmadzadeh A. Conception rates and serum progesterone concentration in dairy cattle administered gonadotropin releasing hormone 5 days after artificial insemination. Anim Reprod Sci. 2006. 95:224–233.
Article12. Kerbler TL, Buhr MM, Jordan LT, Leslie KE, Walton JS. Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle. Theriogenology. 1997. 47:703–714.
Article13. Kuroiwa T, Ishibashi A, Fukuda M, Kim S, Tanaka T, Kamomae H. Estrus synchronization and conception rate after a progesterone releasing intravaginal device (PRID) treatment from the early luteal phase in heifers. J Reprod Dev. 2005. 51:669–673.
Article14. López-Gatius F, Santolaria P, Yániz J, Rutllant J, López-Béjar M. Factors affecting pregnancy loss from gestation day 38 to 90 in lactating dairy cows from a single herd. Theriogenology. 2002. 57:1251–1261.
Article15. Mann GE, Lamming GE. The influence of progesterone during early pregnancy in cattle. Reprod Domest Anim. 1999. 34:269–274.
Article16. Mann GE, Lamming GE, Robinson RS, Wathes DC. The regulation of interferon-tau production and uterine hormone receptors during early pregnancy. J Reprod Fertil Suppl. 1999. 54:317–328.17. Maurer RR, Chenault JR. Fertilization failure and embryonic mortality in parous and non-parous beef cattle. J Anim Sci. 1983. 56:1186–1189.
Article18. Mussard ML, Burke CR, Behlke EJ, Gasser CL, Day ML. Influence of premature induction of a luteinizing hormone surge with gonadotropin-releasing hormone on ovulation, luteal function, and fertility in cattle. J Anim Sci. 2007. 85:937–943.
Article19. Peters KE, Bergfeld EG, Cupp AS, Kojima FN, Mariscal V, Sanchez T, Wehrman ME, Grotjan HE, Hamernik DL, Kittok RJ, Kinder JE. Luteinizing hormone has a role in development of fully functional corpora lutea (CL) but is not required to maintain CL function in heifers. Biol Reprod. 1994. 51:1248–1254.
Article20. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995. 44:915–923.
Article21. Rosenberg M, Kaim M, Herz Z, Folman Y. Comparison of methods for the synchronization of estrous cycles in dairy cows. 1. Effects on plasma progesterone and manifestation of estrus. J Dairy Sci. 1990. 73:2807–2816.
Article22. Santos JEP, Thatcher WW, Chebel RC, Cerri RLA, Galvão KN. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim Reprod Sci. 2004. 82-83:513–535.
Article23. Schafer DJ, Bader JF, Meyer JP, Haden JK, Ellersieck MR, Lucy MC, Smith MF, Patterson DJ. Comparison of progestin-based protocols to synchronize estrus and ovulation before fixed-time artificial insemination in postpartum beef cows. J Anim Sci. 2007. 85:1940–1945.
Article24. Shaham-Albalancy A, Folman Y, Kaim M, Rosenberg M, Wolfenson D. Delayed effect of low progesterone concentrations on bovine uterine PGF2α secretion in the subsequent oestrous cycle. Reproduction. 2001. 122:643–648.25. Tauck SA, Wilkinson JR, Olsen JR, Janitell JN, Berardinelli JG. Comparison of controlled internal drug release device and melengesterol acetate as progestin sources in an estrous synchronization protocol for beef heifers. Theriogenology. 2007. 68:162–167.
Article26. Taya K, Watanabe G, Sasamoto S. Radioimmunoassay for progesterone, testosterone and estradiol-17 beta using 125I-iodohistamine radioligands. Jpn J Anim Reprod. 1985. 31:186–197.
Article27. Thatcher WW, Bilby TR, Bartolome JA, Silvestre F, Staples CR, Santos JEP. Strategies for improving fertility in the modern dairy cow. Theriogenology. 2006. 65:30–44.
Article28. Vasconcelos JLM, Sartori R, Oliveira HN, Guenther JG, Wiltbank MC. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 2001. 56:307–314.
Article29. Walsh RB, Leblanc SJ, Duffield TF, Kelton DF, Walton JS, Leslie KE. The effect of a progesterone releasing intravaginal device (PRID) on pregnancy risk to fixed-time insemination following diagnosis of non-pregnancy in dairy cows. Theriogenology. 2007. 67:948–956.
Article30. Willard S, Gandy S, Bowers S, Graves K, Elias A, Whisnant C. The effects of GnRH administration postinsemination on serum concentrations of progesterone and pregnancy rates in dairy cattle exposed to mild summer heat stress. Theriogenology. 2003. 59:1799–1810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of resynchronization programs according to the ovarian status on pregnancy outcomes in dairy cows
- Effects of gonadotropin-releasing hormone administration or a controlled internal drug-releasing insert after timed artificial insemination on pregnancy rates of dairy cows
- Risk factors for repeat breeder dairy cows and their impacts on reproductive performance
- GNRH test in Male Hypogonadism
- The Effect of GnRH Agonist and Bilateral Oophorectomy on Experimental Endometriosis in the Rabbit