J Vet Sci.
2008 Sep;9(3):247-256. 10.4142/jvs.2008.9.3.247.
Effect of dihydrotestosterone on mouse embryonic stem cells exposed to H(2)O(2)-induced oxidative stress
- Affiliations
-
- 1Department of Urology, Chonnam National University Medical School, Gwangju 501-746, Korea.
- 2Biotherapy Human Resources Center (BK 21), College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea. hjhan@chonnam.ac.kr
- KMID: 1718577
- DOI: http://doi.org/10.4142/jvs.2008.9.3.247
Abstract
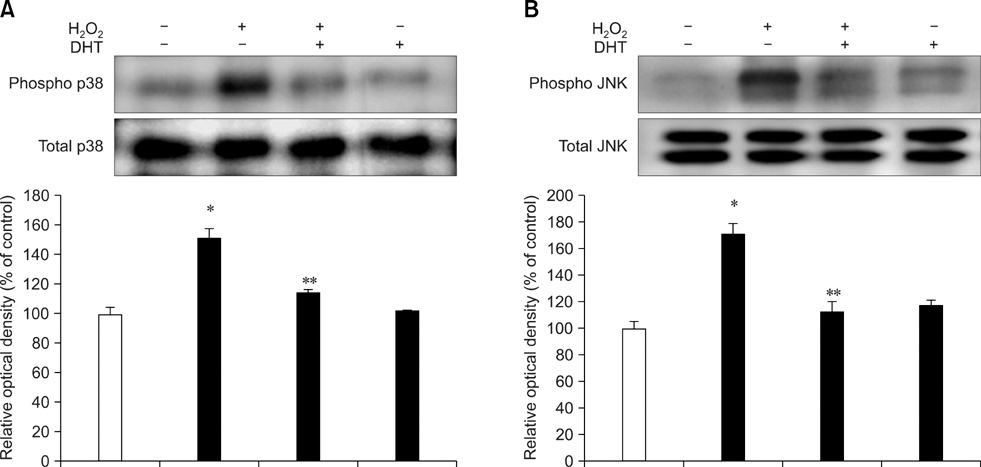
- Oxidative stresses induced by reactive oxygen species (ROS) have been shown to be involved in several physiological and pathophysiological processes, such as cell proliferation and differentiation. Steroid hormones can protect cells against apoptosis or induce cell proliferation by several mechanisms. Among androgenic hormones, dihydrotestosterone (DHT) is generated by a 5alpha- reduction of testosterone. Unlike testosterone, DHT cannot be aromatized to estradiol, therefore DHT is considered a pure androgenic steroid. This study was conducted to examine the effect of DHT (10(-7) M) on H(2)O(2) (10(-3) M) -induced injuries in mouse embryonic stem (ES) cells. H(2)O(2) induced ROS generation and increased lipid peroxide formation and DNA fragmentation. These effects of H(2)O(2) were inhibited by pretreatment with DHT. H(2)O(2) also increased the phosphorylation of p38 MAPK, SAPK/JNK and nuclear factor kappa B (NF-kappaB), but DHT blocked these effects. Moreover, H(2)O(2) decreased DNA synthesis and the levels of cell cycle regulatory proteins [cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 2, and CDK 4]. These effects of H(2)O(2) were inhibited by pretreatment with DHT. In conclusion, DHT may partially prevent H(2)O(2)-induced cell injury through inhibition of ROS and ROS-induced activation of p38 MAPK, SAPK/JNK and NF-kappaB in mouse ES cells.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Cell Culture Techniques
Cells, Cultured
Dihydrotestosterone/*pharmacology
Embryonic Stem Cells/cytology/*drug effects/pathology/*physiology
Enzyme Activation
Hydrogen Peroxide/*pharmacology
Mice
Models, Biological
NF-kappa B/drug effects/metabolism
Oxidative Stress/drug effects/*physiology
Reactive Oxygen Species/metabolism
Signal Transduction/drug effects
Thymidine/metabolism
p38 Mitogen-Activated Protein Kinases/drug effects/metabolism
Figure
Reference
-
1. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005. 3:28.
Article2. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996. 87:13–20.3. Baker AM, Oberley LW, Cohen MB. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate. 1997. 32:229–233.
Article4. Baker MA, Aitken RJ. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol. 2005. 3:67.
Article5. Barnouin K, Dubuisson ML, Child ES, Fernandez de Mattos S, Glassford J, Medema RH, Mann DJ, Lam EW. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem. 2002. 277:13761–13770.
Article6. Baud L, Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986. 251:F765–F776.
Article7. Bennett MJ, Albert RH, Jez JM, Ma H, Penning TM, Lewis M. Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase. Structure. 1997. 5:799–812.
Article8. Bilińska B, Wiszniewska B, Kosiniak-Kamysz K, Kotula-Balak M, Gancarczyk M, Hejmej A, Sadowska J, Marchlewicz M, Kolasa A, Wenda-Rózewicka L. Hormonal status of male reproductive system: androgens and estrogens in the testis and epididymis. In vivo and in vitro approaches. Reprod Biol. 2006. 6:Suppl 1. 43–58.9. Bonvin C, Guillon A, van Bemmelen MX, Gerwins P, Johnson GL, Widmann C. Role of the amino-terminal domains of MEKKs in the activation of NF kappa B and MAPK pathways and in the regulation of cell proliferation and apoptosis. Cell Signal. 2002. 14:123–131.
Article10. Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, Viña J. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NF-κB cascade. Aging Cell. 2005. 4:113–118.
Article11. Brett CM, Washington CB, Ott RJ, Gutierrez MM, Giacomini KM. Interaction of nucleoside analogues with the sodium-nucleoside transport system in brush border membrane vesicles from human kidney. Pharm Res. 1993. 10:423–426.12. Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA. 1997. 94:5709–5712.
Article13. Burton GJ, Charnock-Jones DS, Jauniaux E. Working with oxygen and oxidative stress in vitro. Methods Mol Med. 2006. 122:413–425.14. Bus JS, Aust SD, Gibson JE. Lipid peroxidation: a possible mechanism for paraquat toxicity. Res Commun Chem Pathol Pharmacol. 1975. 11:31–38.15. Carson C 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003. 61:Suppl 1. 2–7.
Article16. Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004. 6:386–391.
Article17. Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995. 270:14843–14846.
Article18. Cochrane CG. Cellular injury by oxidants. Am J Med. 1991. 91:23S–30S.
Article19. Du J, Daniels DH, Asbury C, Venkataraman S, Liu J, Spitz DR, Oberley LW, Cullen JJ. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem. 2006. 281:37416–37426.
Article20. Dumont A, Hehner SP, Hofmann TG, Ueffing M, Droge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene. 1999. 18:747–757.
Article21. Felty Q. Estrogen-induced DNA synthesis in vascular endothelial cells is mediated by ROS signaling. BMC Cardiovasc Disord. 2006. 6:16.
Article22. Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005. 44:6900–6909.
Article23. Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol. 2005. 84:687–697.
Article24. Flores E, Bratoeff E, Cabeza M, Ramirez E, Quiroz A, Heuze I. Steroid 5α-reductase inhibitors. Mini Rev Med Chem. 2003. 3:225–237.25. Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990. 4:2587–2597.
Article26. Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol. 2005. 149:240–260.
Article27. Girotti AW. Photodynamic lipid peroxidation in biological systems. Photochem Photobiol. 1990. 51:497–509.
Article28. Gutierrez-Adan A, Rizos D, Fair T, Moreira PN, Pintado B, de la Fuente J, Boland MP, Lonergan P. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol Reprod Dev. 2004. 68:441–448.29. Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996. 271:4138–4142.30. Han HJ, Heo JS, Lee YJ. Estradiol-17β stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006. 290:C1067–C1075.
Article31. Han HJ, Heo JS, Lee YJ, Min JJ, Park KS. High glucose-induced inhibition of 2-deoxyglucose uptake is mediated by cAMP, protein kinase C, oxidative stress and mitogen-activated protein kinases in mouse embryonic stem cells. Clin Exp Pharmacol Physiol. 2006. 33:211–220.
Article32. Hausburg MA, Dekrey GK, Salmen JJ, Palic MR, Gardiner CS. Effects of paraquat on development of preimplantation embryos in vivo and in vitro. Reprod Toxicol. 2005. 20:239–246.
Article33. Heo JS, Lee YJ, Han HJ. EGF stimulates proliferation of mouse embryonic stem cells: involvement of Ca2+ influx and p44/42 MAPKs. Am J Physiol Cell Physiol. 2006. 290:C123–C133.34. Ikeda M, Hirose Y, Miyoshi K, Kodama H. Nuclear factor kappaB (NF-kappaB) activation by hydrogen peroxide in human epidermal keratinocytes and the restorative effect of interleukin-10. J Dermatol Sci. 2002. 28:159–170.
Article35. Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol. 1998. 10:205–219.
Article36. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995. 270:16483–16486.
Article37. Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity. Oxidant injury without apoptosis. J Biol Chem. 1996. 271:15182–15186.38. Kim YH, Han HJ. Synergistic effect of high glucose and ANG II on proliferation of mouse embryonic stem cells: Involvement of PKC and MAPKs as well as AT1 receptor. J Cell Physiol. 2008. 215:374–382.
Article39. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004. 62:1186–1197.
Article40. Langner C, Ratschek M, Rehak P, Schips L, Zigeuner R. Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol. 2004. 171:611–614.
Article41. Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005. 30:263–270.
Article42. Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002. 406:173–182.
Article43. Maniatis T. Catalysis by a multiprotein IkappaB kinase complex. Science. 1997. 278:818–819.44. Na SI, Lee MY, Heo JS, Han HJ. Hydrogen peroxide increased [3H]-2-deoxyglucose uptake via MAPK, cPLA2, and NF-κB signaling pathway in mouse embryonic stem cells. Cell Physiol Biochem. 2007. 20:1007–1018.
Article45. Negri-Cesi P, Poletti A, Colciago A, Magni P, Martini P, Motta M. Presence of 5α-reductase isozymes and aromatase in human prostate cancer cells and in benign prostate hyperplastic tissue. Prostate. 1998. 34:283–291.
Article46. Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001. 26:137–148.
Article47. O'Shea KS. Embryonic stem cell models of development. Anat Rec. 1999. 257:32–41.48. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article49. Park BG, Yoo CI, Kim HT, Kwon CH, Kim YK. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology. 2005. 215:115–125.
Article50. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004. 11:1163–1182.
Article51. Prelle K, Zink N, Wolf E. Pluripotent stem cells-model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002. 31:169–186.
Article52. Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst. 1997. 89:40–48.
Article53. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995. 9:1149–1163.
Article54. Sigaud S, Evelson P, González-Flecha B. H2O2-induced proliferation of primary alveolar epithelial cells is mediated by MAP kinases. Antioxid Redox Signal. 2005. 7:6–13.
Article55. Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990. 8:583–599.
Article56. Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995. 80:529–532.57. Wang D, Richmond A. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001. 276:3650–3659.
Article58. Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998. 333:291–300.
Article59. Ward PA, Warren JS, Johnson KJ. Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med. 1988. 5:403–408.
Article60. Wolf R, Schönfelder G, Paul M, Blume-Peytavi U. Nitric oxide in the human hair follicle: constitutive and dihydrotestosterone-induced nitric oxide synthase expression and NO production in dermal papilla cells. J Mol Med. 2003. 81:110–117.
Article61. Yin DM, Wu JC, Yuan YJ. Reactive oxygen species, cell growth, and taxol production of Taxus cuspidata cells immobilized on polyurethane foam. Appl Biochem Biotechnol. 2005. 127:173–185.62. Zhu Z, Mukhina S, Zhu T, Mertani HC, Lee KO, Lobie PE. p44/42 MAP kinase-dependent regulation of catalase by autocrine human growth hormone protects human mammary carcinoma cells from oxidative stress-induced apoptosis. Oncogene. 2005. 24:3774–3785.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isoflurane decreases apoptosis of human embryonic stem cell-derived cardiac progenitor cell under oxidative stress
- Problems Associated with Establishment of Human Embryonic Stem Cell
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Melatonin mitigates the adverse effect of hypoxia during myocardial differentiation in mouse embryonic stem cells
- Expression of CD44 in mouse diethylnitrosamine (DEN)-induced hepatic tumors as well as in DEN-treated embryo stem cells and derived hepatic lineage cells