Korean J Gastroenterol.
2011 Apr;57(4):221-229. 10.4166/kjg.2011.57.4.221.
Regional Difference of Antibiotic Resistance of Helicobacter pylori Strains in Korea
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea. nayoungkim49@empal.com
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 4Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 5Department of Microbiology, Hanyang University School of Medicine, Seoul, Korea.
- KMID: 1718402
- DOI: http://doi.org/10.4166/kjg.2011.57.4.221
Abstract
- BACKGROUND/AIMS
This study was performed to compare the prevalence rates of primary antibiotic resistance in Helicobacter pylori (H. pylori) isolates among different regions of Korea.
METHODS
H. pylori were isolated from gastric mucosal biopsy specimens of 99 Koreans who lived in Gyeonggi (n=40), Kangwon province (n=40) and Busan (n=19) from April to August in 2008. All the patients had no history of H. pylori eradication therapy. The susceptibilities of the H. pylori isolates to amoxicillin, clarithromycin, metronidazole, tetracycline, azithromycin, ciprofloxacin, levofloxacin, and moxifloxacin were tested according to the agar dilution method.
RESULTS
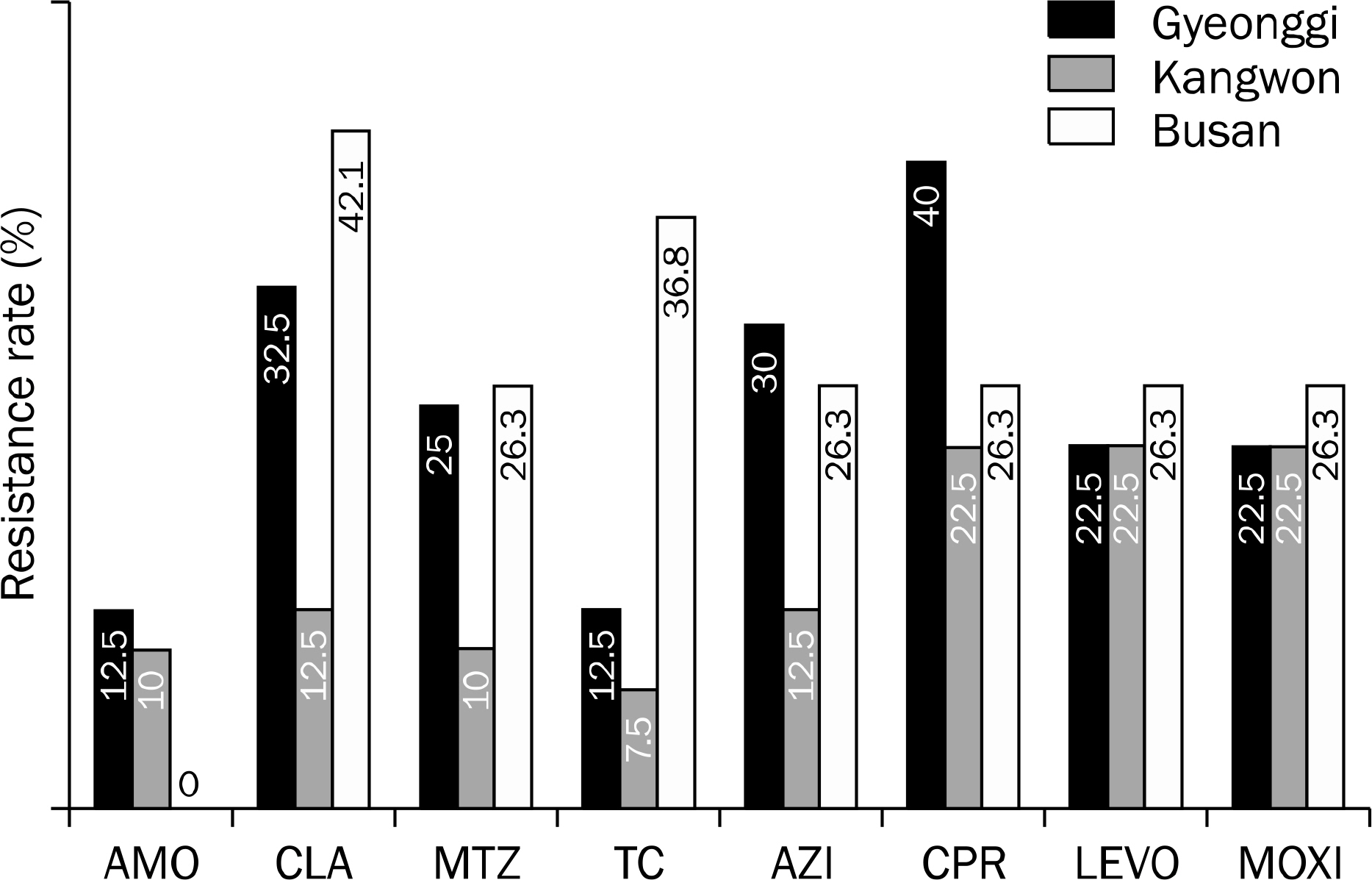
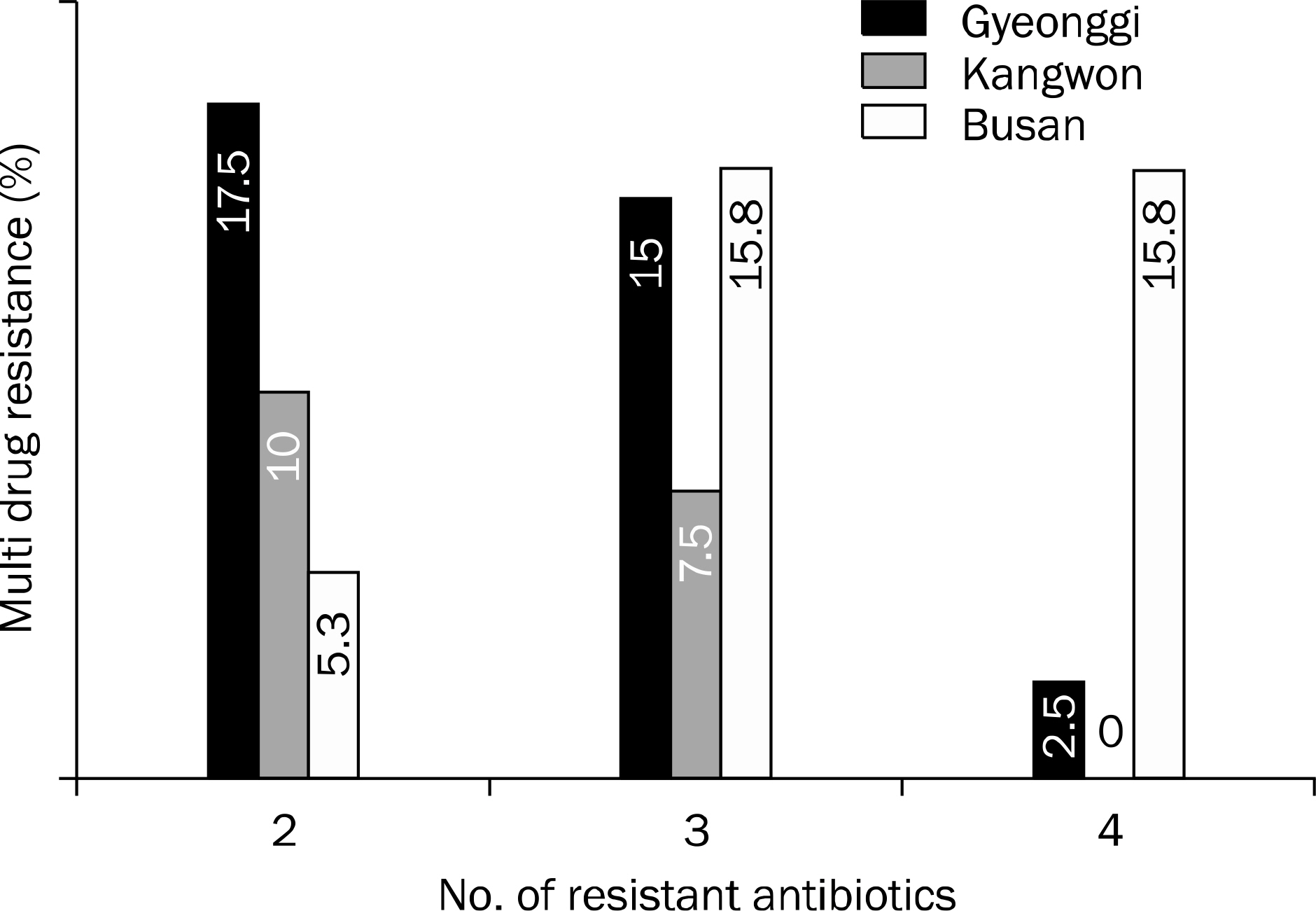
There was a difference in resistance to clarithromycin in three institutes located among Gyeonggi (32.5%), Kangwon province (12.5%) and Busan (42.1%) by One way ANOVA test (p=0.027) and nonparametric Kruskal Wallis test (p=0.027). However, by post-hoc analysis, there was no statistically significant difference among three regions. Similarly, the other 7 antibiotics (amoxicillin, metronidazole, tetracycline, azithromycin, ciprofloxacin, levofloxacin and moxifloxacin) did not show any significant difference.
CONCLUSIONS
There was no significant regional difference of the primary antibiotic resistance of H. pylori. However, the included patient number might not be enough for this conclusion demanding further evaluations.
Keyword
MeSH Terms
-
Amoxicillin/pharmacology
Anti-Bacterial Agents/pharmacology/therapeutic use
Aza Compounds/pharmacology
Azithromycin/pharmacology
Ciprofloxacin/pharmacology
Clarithromycin/pharmacology
*Drug Resistance, Bacterial
Female
Helicobacter Infections/*epidemiology/microbiology
Helicobacter pylori/*drug effects/isolation & purification
Humans
Male
Metronidazole/pharmacology
Microbial Sensitivity Tests
Middle Aged
Ofloxacin/pharmacology
Quinolines/pharmacology
Republic of Korea/epidemiology
Tetracycline/pharmacology
Figure
Reference
-
References
1. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994; 272:65–69.2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.3. Malfertheiner P, Mégraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection–the Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002; 16:167–180.4. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56:772–781.5. Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection–a metaanalysis. Aliment Pharmacol Ther. 1999; 13:857–864.6. Cho EJ, Lee DH, Chun JY, et al. Recent trends in the eradication rates of second-line quadruple therapy for Helicobacter pylori and the clinical factors that potentially affect the treatment outcome. Korean J Gastrointest Endosc. 2009; 38:14–19.7. Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007; 45:4006–4010.8. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.9. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:321–331.10. Kato M, Yamaoka Y, Kim JJ, et al. Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob Agents Chemother. 2000; 44:2214–2216.11. Queiroz DM, Dani R, Silva LD, et al. Factors associated with treatment failure of Helicobacter pylori infection in a developing country. J Clin Gastroenterol. 2002; 35:315–320.12. Mégraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004; 53:1374–1384.13. Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006; 40:683–687.14. Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Alterations in pen-icillin-binding protein 1A confer resistance to beta-lactam antibiotics in Helicobacter pylori. Antimicrob Agents Chemother. 2002; 46:2229–2233.15. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.16. Kwon DH, Dore MP, Kim JJ, et al. High-level beta-lactam resistance associated with acquired multidrug resistance in Helicobacter pylori. Antimicrob Agents Chemother. 2003; 47:2169–2178.17. Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar CM, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997; 41:2724–2728.18. Kim JJ, Reddy R, Lee M, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001; 47:459–461.19. Toracchio S, Marzio L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998–2002. Dig Liver Dis. 2003; 35:541–545.20. Pilotto A, Rassu M, Leandro G, Franceschi M, Di Mario F. Interdisciplinary Group for the Study of Ulcer. Prevalence of Helicobacter pylori resistance to antibiotics in Northeast Italy: a multicentre study. GISU. Interdisciplinary Group for the Study of Ulcer. Dig Liver Dis. 2000; 32:763–768.21. Mégraud F. Basis for the management of drug-resistant Helicobacter pylori infection. Drugs. 2004; 64:1893–1904.22. Park MJ, Choi IJ, Kim JS, et al. Efficacy of quadruple therapy as retreatment regimen in Helicobacter pylori-positive peptic ulcer disease. Korean J Gastroenterol. 2000; 36:457–464.23. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.24. Ivashkin VT, Lapina TL, Bondarenko OY, et al. Azithromycin in a triple therapy for H. pylori eradication in active duodenal ulcer. World J Gastroenterol. 2002; 8:879–882.25. Gisbert JP, Morena F. Systematic review and metaanalysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006; 23:35–44.26. Li Y, Huang X, Yao L, Shi R, Zhang G. Advantages of Moxifloxacin and Levofloxacin-based triple therapy for second-line treatments of persistent Helicobacter pylori infection: a meta analysis. Wien Klin Wochenschr. 2010; 122:413–422.27. Yoon H, Kim N, Lee BH, et al. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009; 14:77–85.28. Chen LW, Chien RN, Chang JJ, Fang KM, Chang LC. Comparison of the once-daily levofloxacin-containing triple therapy with the twice-daily standard triple therapy for first-line Helicobacter pylori eradication: a prospective randomised study. Int J Clin Pract. 2010; 64:1530–1534.29. Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003; 17:719–726.30. Scaccianoce G, Hassan C, Panarese A, Piglionica D, Morini S, Zullo A. Helicobacter pylori eradication with either 7-day or 10-day triple therapies, and with a 10-day sequential regimen. Can J Gastroenterol. 2006; 20:113–117.31. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007; 146:556–563.32. Paoluzi OA, Visconti E, Andrei F, et al. Ten and eight-day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: a randomized controlled study on efficacy and tolerability. J Clin Gastroenterol. 2010; 44:261–266.33. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008; 148:923–931.34. Kwon JH, Lee DH, Song BJ, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010; 15:148–153.35. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.36. Park S, Chun HJ, Kim ES, et al. The 10-day sequential therapy for Helicobacter pylori eradication in Korea: less effective than expected. Gastroenterology. 2009; 136:A–339. A-340.37. Kim YS, Kim N, Kim JM, et al. Helicobacter pylori genotyping findings from multiple cultured isolates and mucosal biopsy specimens: strain diversities of Helicobacter pylori isolates in individual hosts. Eur J Gastroenterol Hepatol. 2009; 21:522–528.38. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006; 47:337–349.39. Kim JJ, Kim JG, Kwon DH. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003; 8:202–206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications
- Antibiotic Resistance of Helicobacter pylori Isolated from Korean Patients
- Clinical Characteristicsof Patients with Failed Eradication of Helicobacter pylori and Antibiotic Resistance
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance
- Antibiotic Resistance in Helicobacter pylori