Yonsei Med J.
2012 Jul;53(4):849-855. 10.3349/ymj.2012.53.4.849.
Anococcygeal Raphe Revisited: A Histological Study Using Mid-Term Human Fetuses and Elderly Cadavers
- Affiliations
-
- 1Division of Colon and Rectal Surgery, Shizuoka Cancer Center Hospital, Shizuoka, Japan. y.kinugasa@scchr.jp
- 2Arakawa Clinic of Proctology, Tokyo, Japan.
- 3Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 4Oral Health Science Center hrc8, Tokyo Dental College, Chiba, Japan.
- 5Department of Surgery, Chonbuk National University School of Medicine, Jeonju, Korea.
- 6Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 7Department of Surgical Oncology, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan.
- KMID: 1716888
- DOI: http://doi.org/10.3349/ymj.2012.53.4.849
Abstract
- PURPOSE
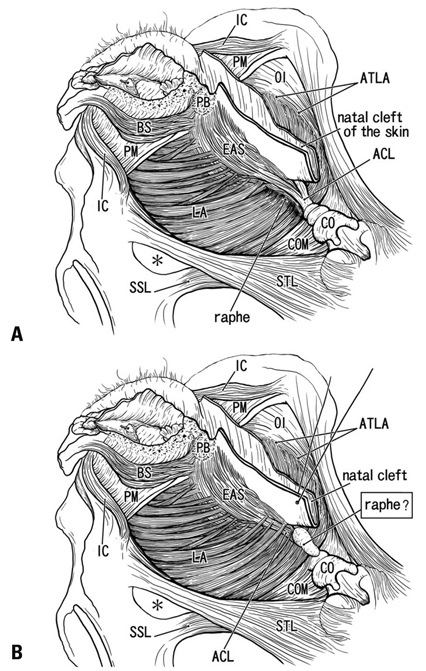
We recently demonstrated the morphology of the anococcygeal ligament. As the anococcygeal ligament and raphe are often confused, the concept of the anococcygeal raphe needs to be re-examined from the perspective of fetal development, as well as in terms of adult morphology.
MATERIALS AND METHODS
We examined the horizontal sections of 15 fetuses as well as adult histology. From cadavers, we obtained an almost cubic tissue mass containing the dorsal wall of the anorectum, the coccyx and the covering skin. Most sections were stained with hematoxylin and eosin or Masson-trichrome solution.
RESULTS
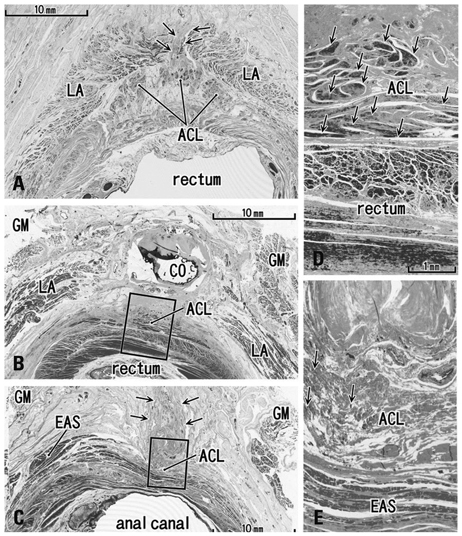
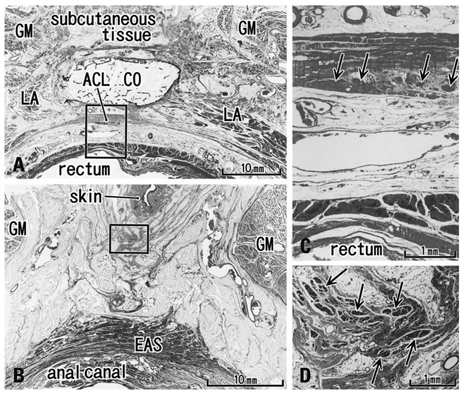
The adult ligament contained both smooth and striated muscle fibers. A similar band-like structure was seen in fetuses, containing: 1) smooth muscle fibers originating from the longitudinal muscle coat of the anal canal and 2) striated muscle fibers from the external anal sphincter (EAS). However, in fetuses, the levator ani muscle did not attach to either the band or the coccyx. Along and around the anococcygeal ligament, we did not find any aponeurotic tissue with transversely oriented fibers connecting bilateral levator ani slings. Instead, in adults, a fibrous tissue mass was located at a gap between bilateral levator ani slings; this site corresponded to the dorsal side of the ligament and the EAS in the immediately deep side of the natal skin cleft.
CONCLUSION
We hypothesize that a classically described raphe corresponds to the specific subcutaneous tissue on the superficial or dorsal side of the anococcygeal ligament.
Keyword
MeSH Terms
Figure
Reference
-
1. Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, et al. Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 2011. 54:232–237.
Article2. Bogduk N. Issues in anatomy: the external anal sphincter revisited. Aust N Z J Surg. 1996. 66:626–629.
Article3. Borley NR. Standring S, editor. Anal canal. Gray's Anatomy. 2008. 40 ed. London: Elsevier Churchill Linvingstone;1155–1160.4. Toldt BvC. Atlas of Human Anatomy for Students and Surgeons. 1903. Berlin: Urban & Schwarzenberg.5. Gräfenberg E. Development of the human pelvic musculature. Anat Hefte. 1904. 72:429–494.6. Bardeen RC. Development and variation of the musculature of the inferior extremity and the neighboring regions of the trunk in man. Am J Anat. 1907. 6:332–336.7. Power RM. Embryological development of the levator ani muscle. Am J Obstet Gynecol. 1948. 55:367–381.
Article8. Tichý M. The development and organization of the sphincter ani externus and the adjacent part of the levator ani muscle in man. Folia Morphol (Praha). 1984. 32:113–120.9. Fritsch H, Fröhlich B. Development of the levator ani muscle in human fetuses. Early Hum Dev. 1994. 37:15–25.
Article10. Fröber R, Krebs U, Haas A, Fischer MS, Schier F, Linss W. Three-dimensional reconstruction of the anal striated musculature in a human fetus. Cells Tissues Organs. 2001. 169:152–157.
Article11. Schier F, Krebs U, Fröber R, Haas A. Three-dimensional reconstruction of the anorectal continence organ in a 14-week-old fetus. J Pediatr Surg. 2002. 37:912–915.
Article12. Koch WF, Marani E. Early development of the human pelvic diaphragm. Adv Anat Embryol Cell Biol. 2007. 192:1–111.
Article13. Fritsch H. Developmental changes in the retrorectal region of the human fetus. Anat Embryol (Berl). 1988. 177:513–522.
Article14. Levi AC, Borghi F, Garavoglia M. Development of the anal canal muscles. Dis Colon Rectum. 1991. 34:262–266.
Article15. Niikura H, Jin ZW, Cho BH, Murakami G, Yaegashi N, Lee JK, et al. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010. 23:566–574.
Article16. Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008. 15:640–647.
Article17. Miyake N, Hayashi S, Kawase T, Cho BH, Murakami G, Fujimiya M, et al. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec (Hoboken). 2010. 293:438–445.
Article18. Ayoub SF. The anterior fibres of the levator ani muscle in man. J Anat. 1979. 128(Pt 3):571–580.19. Ayoub SF. Anatomy of the external anal sphincter in man. Acta Anat (Basel). 1979. 105:25–36.
Article20. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, et al. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.
Article21. Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation). Okajimas Folia Anat Jpn. 2010. 87:49–58.
Article22. Henrich M. Clinical topography of the proctodeum. Acta Anat (Basel). 1980. 106:161–170.
Article23. Courtney H. Anatomy of the pelvic diaphragm and anorectal musculature as related to sphincter preservation in anorectal surgery. Am J Surg. 1950. 79:155–173.
Article24. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, et al. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004. 79:72–81.
Article25. Shafik A. New concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. II. Anatomy of the levator ani muscle with special reference to puborectalis. Invest Urol. 1975. 13:175–182.26. Shafik A. Levator ani muscle: new physioanatomical aspects and role in the micturition mechanism. World J Urol. 1999. 17:266–273.
Article27. García-Armengol J, García-Botello S, Martinez-Soriano F, Roig JV, Lledó S. Review of the anatomic concepts in relation to the retrorectal space and endopelvic fascia: waldeyer's fascia and the rectosacral fascia. Colorectal Dis. 2008. 10:298–302.
Article28. McKirdy HC. Anatomy and function of the anal longitudinal muscle. Br J Surg. 1993. 80:262.
Article29. Olson L, Alund M. Quinacrine-binding nerves: presence in the mouse ano-coccygeus muscle, disappearance after muscle transsection. Med Biol. 1979. 57:182–186.30. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, et al. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011. 37:13–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perineal raphe with special reference to its extension to the anus: a histological study using human fetuses
- The effect of treatment with tryptophan and/or reserpine on the serotonergic immunoreactivity in raphe nucleus of medulla oblongata and midbrain of the rats
- A temporary disc-like structure at the median atlanto-axial joint in human fetuses
- Distally-extending muscle fibers across involved joints: study of long muscles and tendons of wrist and ankle in late-term fetuses and adult cadavers
- Embryologic Discission of the Median Raphe Cyst: Two Cases Report