J Korean Med Sci.
2010 May;25(5):723-727. 10.3346/jkms.2010.25.5.723.
Effects of Cyclosporin A Therapy Combined with Steroids and Angiotensin Converting Enzyme Inhibitors on Childhood IgA Nephropathy
- Affiliations
-
- 1The Institute of Kidney Disease, Department of Pediatrics, Yonsei University College of Medicine, Severance Children's Hospital, Seoul, Korea. kkkjhd@yuhs.ac
- 2Department of Pathology, Chungnam National University College of Medicine, Daejeon, Korea.
- 3Department of Pediatrics, Kwangdong University College of Medicine, Goyang, Korea.
- 4The Institute of Kidney Disease, Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1713956
- DOI: http://doi.org/10.3346/jkms.2010.25.5.723
Abstract
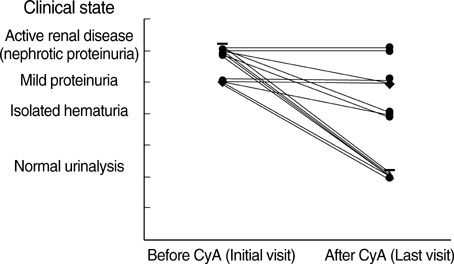
- To evaluate the effects of cyclosporin A (CyA) on clinical outcome and pathologic changes in children with IgA nephropathy (IgAN), we retrospectively evaluated 14 children (mean age 8.9+/-2.9 yr; eight males, six females) who were treated with CyA and steroids. The starting dose of CyA was 5 mg/kg per day, and the drug level was maintained at 100-200 ng/mL. The mean CyA level was 183.8+/-48.3 ng/mL (range 120.7-276.0 ng/mL) and the mean duration of CyA therapy was 10.9+/-1.9 months (range 8-12 months). After CyA therapy the mean 24 hr urinary protein excretion declined from 107.1+/-35.1 mg/m2/hr to 7.4+/-2.4 mg/m2/hr (P<0.001) and serum albumin increased from 3.3+/-0.6 g/dL to 4.3+/-0.3 g/dL (P<0.001). At a follow-up biopsy the histological grade of IgAN was improved in seven (50%) of the 14 patients, remained the same in three (21%), and was aggravated in four (29%). Serum creatinine, creatinine clearance, and blood pressure did not differ before and after CyA therapy. Two patients (14%) showed CyA-induced nephrotoxicity at the second biopsy. Our findings indicate that CyA therapy may be effective in reducing proteinuria and regressing renal pathology in a subset of children with IgAN.
MeSH Terms
-
Angiotensin-Converting Enzyme Inhibitors/*administration & dosage
Child
Cyclosporine/*administration & dosage
Drug Combinations
Female
Glomerulonephritis, IGA/*diagnosis/*drug therapy
Humans
Immunosuppressive Agents/administration & dosage
Male
Steroids/*administration & dosage
Treatment Outcome
Angiotensin-Converting Enzyme Inhibitors
Drug Combinations
Immunosuppressive Agents
Steroids
Cyclosporine
Figure
Reference
-
1. Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris). 1968. 74:694–695.2. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002. 347:738–748.
Article3. D'Amico G. Natural history of idiopathic IgA nephropathy: the role of clinical and histological prognostic factors. Am J Kidney Dis. 2002. 36:227–237.4. Yoshikawa N, Tanaka R, Iijima K. Pathophysiology and treatment of IgA nephropathy in children. Pediatr Nephrol. 2001. 16:446–457.
Article5. Klein M, Radhakrishnan J, Appel G. Cyclosporine treatment of glomerular diseases. Annu Rev Med. 1999. 50:1–15.6. Lai KN, Lai FM, Li PK, Vallance-Owen J. Cyclosporin treatment of IgA nephropathy: a short term controlled trial. Br Med J. 1987. 295:1165–1168.
Article7. Chabova V, Tesar V, Zabka J, Rychlik I, Merta M, Jirsa M Jr, Stejskalová A. Long-term treatment of IgA nephropathy with cyclosporin A--a preliminary report. Nephrol Dial Transplant. 1997. 12:2206–2207.
Article8. Kim PK, Kim KS, Pai KS, Kim JH, Choi IJ. Long-term results of cyclosporine-induced remission of relapsing nephrotic syndrome in children. Yonsei Med J. 1997. 38:307–318.
Article9. Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997. 29:829–842.
Article10. Andreoli SP, Bergstein JM. Treatment of severe IgA nephropathy in children. Pediatr Nephrol. 1989. 3:248–253.
Article11. Foster BJ, Bernard C, Drummond KN, Sharma AK. Effective therapy for severe Henoch-Schönlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr. 2000. 136:370–375.12. Meadow SR, Glasgow EF, White RHR, Moncrief MW, Cameron JS, Ogg CS. Schünlein-Henoch nephritis. Q J Med. 1972. 41:241–258.13. Barratt J, Feehally J. IgA Nephropathy. J Am Soc Nephrol. 2005. 16:2088–2097.
Article14. Goumenos DS, Brown CB. Therapeutic approach of patients with IgA nephropathy. Ren Fail. 2004. 26:171–177.
Article15. Chan JC, Trachtman H. Modulating the progression in IgA nephropathy. Nephron Clin Pract. 2006. 104:c61–c68.
Article16. Meyrier A. Ciclosporin in the treatment of nephrosis. Minimal change disease and focal-segmental glomerulosclerosis. Am J Nephrol. 1989. 9:Suppl 1. 65–71.17. Maruyama K, Tomizawa S, Seki Y, Arai H, Kuroume T. Inhibition of vascular permeability factor production by ciclosporin in minimal change nephrotic syndrome. Nephron. 1992. 62:27–30.
Article18. Zietse R, Wenting GJ, Kramer P, Schalekamp MA, Weimar W. Effects of cyclosporin A on glomerular barrier function in the nephrotic syndrome. Clin Sci (Lond). 1992. 82:641–650.
Article19. Chung WY, Lim IS, Lee SY, Lee SK. Immunologic and morphologic study of experimental IgA nephropathy in ddY mice after administration of cyclosporin A (CyA) (Abstract). Kidney Int. 1992. 41:1952–1953.20. Allen AC, Layward L, Harper SJ, Feehally J. In vitro immunoglobulin isotype suppression in immunoglobulin A nephropathy. Exp Nephrol. 1994. 2:166–170.21. Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2009. 53:5–8.
Article22. Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, Ito H. Japanese Pediatric IgA Nephropathy Treatment Study Group. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006. 1:511–517.
Article23. Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, Kirschstein M, Linné T. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007. 18:1880–1888.
Article24. Tikkanen I, Johnston CI. Comparison of renin-angiotensin to calcium channel blockade in renal disease. Kidney Int Suppl. 1997. 63:Suppl. S19–S22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Angiotensin Converting Enzyme Inhibitors on Induced Angiotensin Converting Enzyme Activity in Rat Intestine
- Renoprotective effect of deflazacort in IgA nephropathy with proteinuria
- Angiotensin Converting Enzyme Inhibitors for the
- ACE Inhibitors and Losartan in the Managerment of Hypertesion
- A Case of Overlapping Syndrome of Primary IgA Nephropathy and Membranous Nephropathy