J Korean Med Sci.
2010 Jan;25(1):54-60. 10.3346/jkms.2010.25.1.54.
Long-term Treatment Outcomes for Autoimmune Hepatitis in Korea
- Affiliations
-
- 1Department of Medicine, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. liverjhlee@skku.edu
- KMID: 1713832
- DOI: http://doi.org/10.3346/jkms.2010.25.1.54
Abstract
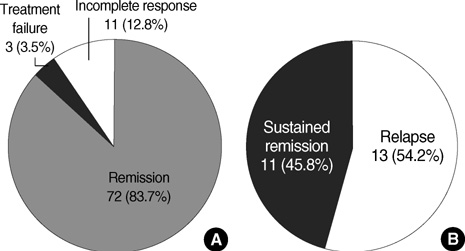
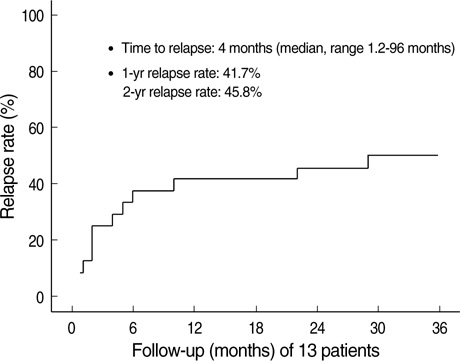
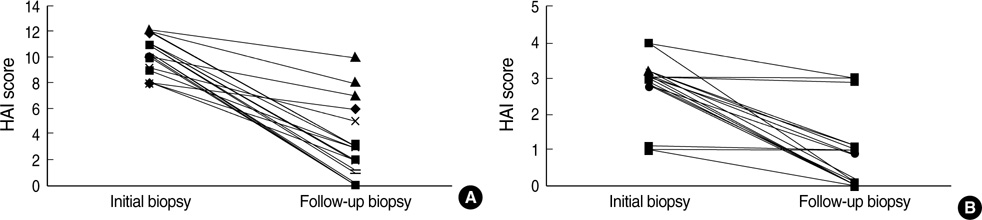
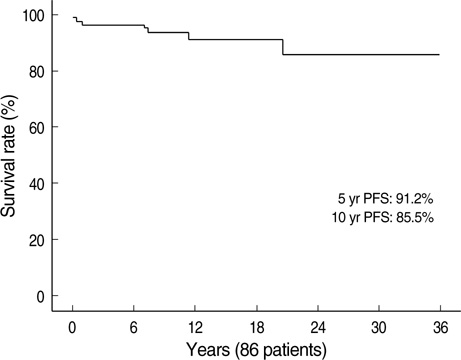
- Immunosuppressive therapy can improve clinical, biochemical and histological features and considerably prolong survival in patients with autoimmune hepatitis. Although ethnicity may affect disease severity and presentation, the long-term outcome of immunosuppression in Korean populations is unknown. This study was aimed to assess the efficacy of immunosuppressive therapy and determine the prognosis of autoimmune hepatitis in Korean populations. We reviewed the medical records of 86 patients diagnosed as having autoimmune hepatitis at the Samsung Medical Center between 1994 and 2008. Seventy-two (83.7%) patients reached remission after a median treatment duration of 3.5 months (range 1 to 44 months). Attempts to withdraw medications were made in 24 cases after the median treatment duration of 36 months (median 6 to 125 months). Thirteen of 24 (54.1%) patients relapsed after treatment withdrawal. Of the 86 patients, 6 (7.2%) experienced disease progression and the overall 5-and 10-yr progression-free survival rates were 91.2% and 85.5%, respectively. In conclusion, immunosuppressive therapy for autoimmune hepatitis results in a favorable rate of remission and excellent progression-free survival, but the relapse rate after treatment withdrawal is high. This suggests that long-term immunosuppressive therapy may be particularly important for treatment of Korean patients.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Azathioprine/therapeutic use
Disease-Free Survival
Drug Therapy, Combination
Female
Hepatitis, Autoimmune/*drug therapy/mortality/pathology
Humans
Immunosuppressive Agents/*therapeutic use
Male
Middle Aged
Prednisolone/therapeutic use
Prognosis
Recurrence
Republic of Korea
Retrospective Studies
Time Factors
Treatment Outcome
Immunosuppressive Agents
Azathioprine
Prednisolone
Figure
Cited by 2 articles
-
A Case of Autoimmune Hepatitis after Occupational Exposure to N,N-Dimethylformamide
Boo-ok Jang, Gwang Hyeon Choi, Hee Yoon Jang, Soomin Ahn, Jae Kyun Choi, Siho Kim, Kyunghan Lee, Eun Sun Jang, Jin-Wook Kim, Sook-Hyang Jeong
J Korean Med Sci. 2020;35(28):e228. doi: 10.3346/jkms.2020.35.e228.Treatment of Autoimmune Hepatitis
Ja Kyung Kim
Korean J Gastroenterol. 2023;81(2):72-85. doi: 10.4166/kjg.2023.011.
Reference
-
1. Czaja AJ, Ammon HV, Summerskill WH. Clinical features and prognosis of severe chronic active liver disease (CALD) after corticosteroid-induced remission. Gastroenterology. 1980. 78:518–523.
Article2. Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006. 354:54–66.
Article3. Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972. 63:820–833.
Article4. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002. 36:479–497.
Article5. Czaja AJ, Doherty DG, Donaldson PT. Genetic bases of autoimmune hepatitis. Dig Dis Sci. 2002. 47:2139–2150.6. Donaldson PT, Czaja AJ. Genetic effects on susceptibility, clinical expression, and treatment outcome of type 1 autoimmune hepatitis. Clin Liver Dis. 2002. 6:707–725.
Article7. Hegarty JE, Nouri Aria KT, Portmann B, Eddleston AL, Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983. 3:685–689.
Article8. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Buschenfelde KH, Mieli-Vergani G, Nakanuma Y, Nishioka M, Penner E, Porta G, Portmann BC, Reed WD, Rodes J, Schalm SW, Scheuer PJ, Schrumpf E, Seki T, Toda G, Tsuji T, Tygstrup N, Vergani D, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999. 31:929–938.
Article9. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002. 6:635–647.10. Toda G, Zeniya M, Watanabe F, Imawari M, Kiyosawa K, Nishioka M, Tsuji T, Omata M. Present status of autoimmune hepatitis in Japan--correlating the characteristics with international criteria in an area with a high rate of HCV infection. Japanese National Study Group of Autoimmune Hepatitis. J Hepatol. 1997. 26:1207–1212.11. Lee YS. Autoimmune hepatitis: recent update on diagnosis and treatment. Korean J Hepatol. 2006. 12:318–332.12. Czaja AJ, Donaldson PT. Genetic susceptibilities for immune expression and liver cell injury in autoimmune hepatitis. Immunol Rev. 2000. 174:250–259.
Article13. Lim KN, Casanova RL, Boyer TD, Bruno CJ. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol. 2001. 96:3390–3394.
Article14. Lim YS, Oh HB, Choi SE, Kwon OJ, Heo YS, Lee HC, Suh DJ. Susceptibility to type 1 autoimmune hepatitis is associated with shared amino acid sequences at positions 70-74 of the HLA-DRB1 molecule. J Hepatol. 2008. 48:133–139.
Article15. Omagari K, Kinoshita H, Kato Y, Nakata K, Kanematsu T, Kusumoto Y, Mori I, Furukawa R, Tanioka H, Tajima H, Koga M, Yano M, Kohno S. Clinical features of 89 patients with autoimmune hepatitis in Nagasaki Prefecture, Japan. J Gastroenterol. 1999. 34:221–226.
Article16. Jung SH, Kim BH, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R. Clinical features of Korean patients with autoimmune hepatitis diagnosed since 1991. Korean J Gastroenterol. 2001. 37:362–369.17. Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984. 4:622–627.
Article18. Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology. 1988. 95:448–453.
Article19. Czaja AJ, Menon KV, Carpenter HA. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: a retrospective analysis. Hepatology. 2002. 35:890–897.
Article20. Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007. 27:507–515.
Article21. Seo S, Toutounjian R, Conrad A, Blatt L, Tong MJ. Favorable outcomes of autoimmune hepatitis in a community clinic setting. J Gastroenterol Hepatol. 2008. 23:1410–1414.
Article22. Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007. 102:1005–1012.
Article23. Kanzler S, Gerken G, Lohr H, Galle PR, Meyer zum Buschenfelde KH, Lohse AW. Duration of immunosuppressive therapy in autoimmune hepatitis. J Hepatol. 2001. 34:354–355.
Article24. Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004. 99:1510–1516.
Article25. Miyake Y, Iwasaki Y, Terada R, Takagi S, Okamaoto R, Ikeda H, Sakai N, Makino Y, Kobashi H, Takaguchi K, Sakaguchi K, Shiratori Y. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. J Hepatol. 2005. 43:951–957.
Article26. Schvarcz R, Glaumann H, Weiland O. Survival and histological resolution of fibrosis in patients with autoimmune chronic active hepatitis. J Hepatol. 1993. 18:15–23.
Article27. Masaki N, Hayashi S. Hepatocellular carcinoma complicating autoimmune hepatitis. Nippon Rinsho. 2001. 59:Suppl 6. 455–464.28. Hardee JT, Breth GF, El-Serag HB. Hepatocellular carcinoma associated with autoimmune hepatitis. J Clin Gastroenterol. 2003. 37:271–272.
Article29. Kanzler S, Lohr H, Gerken G, Galle PR, Lohse AW. Long-term management and prognosis of autoimmune hepatitis (AIH): a single center experience. Z Gastroenterol. 2001. 39:339–341.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclosporine Treatment in a Patient with Concurrent Autoimmune Urticaria and Autoimmune Hepatitis
- Long Term Outcomes after Pediatric Liver Transplantation
- A Case of Autoimmune Hepatitis in Postmenopausal Woman
- Clinical Characteristics, Histology and Prognosis of Autoimmune Hepatitis in Korean Children
- A Case of Autoimmune Hepatitis