J Korean Med Sci.
2007 Oct;22(5):898-904. 10.3346/jkms.2007.22.5.898.
Transtorming Growth Factor beta1 Induces Epithelial-to-Mesenchymal Transition of A549 Cells
- Affiliations
-
- 1Department of Internal Medicine, Hallym University Sacred Heart Hospital, College of Medicine, Hallym University, Anyang, Korea. dongyu@hallym.ac.kr
- KMID: 1713300
- DOI: http://doi.org/10.3346/jkms.2007.22.5.898
Abstract
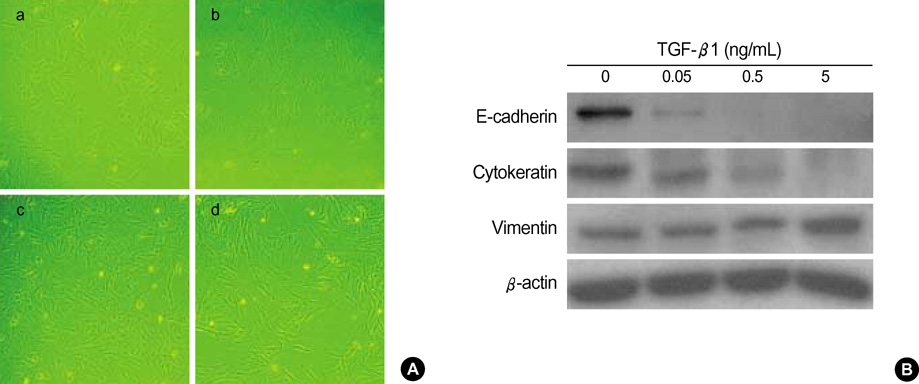
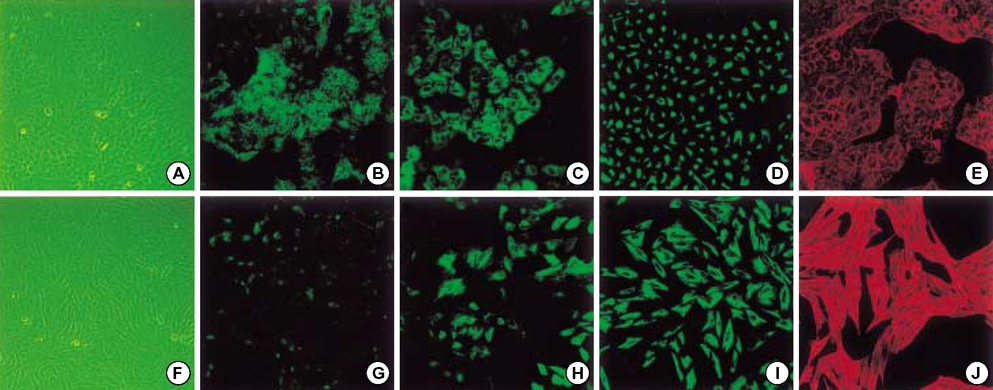
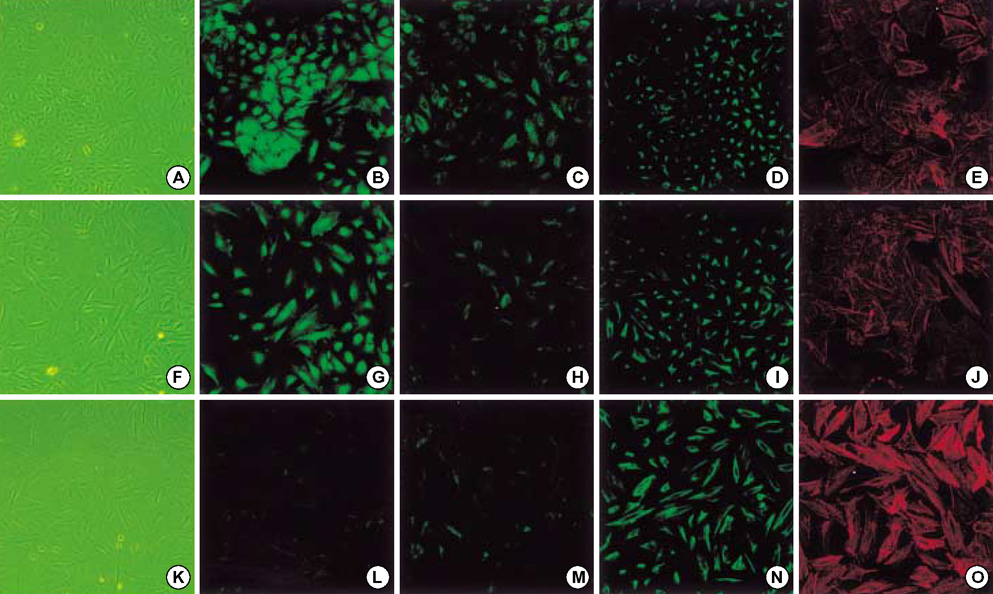
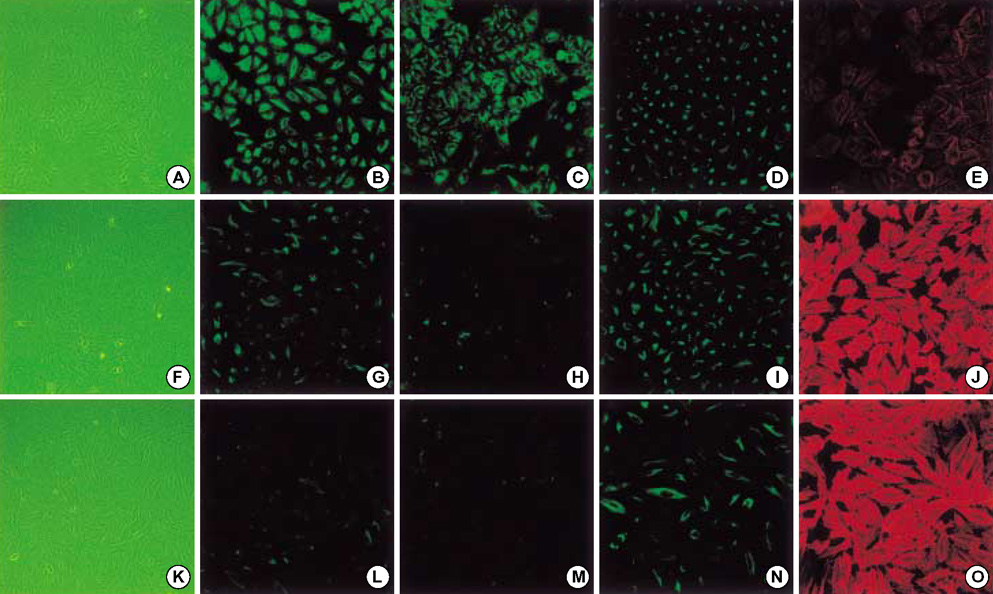
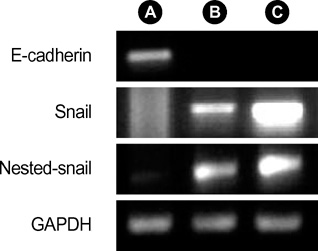
- Idiopathic pulmonary fibrosis (IPF) comprises an aggregate of mesenchymal cells. However, the cellular origin of these mesenchymal phenotypes remains unclear. Transforming growth factor beta1 (TGF-beta1) has been known as the main cytokine involved in the pathogenesis of IPF. We examined whether the potent fibrogenic cytokine TGF-beta1 could induce the epithelial-to-mesenchymal transition (EMT) in the human alveolar epithelial cell line, A549, and determined whether snail expression is associated with the phenotypic changes observed in the A549 cells. EMT was investigated with cells morphology changes under phase-contrast microscopy, western blotting, and indirect immunofluorescence stains. E-cadherin and transcription factor, snail, were also evaluated by measuring mRNA levels using reverse transcriptase-polymerase chain rection (RT-PCR) analysis. The data showed that TGF-beta1 induced A549 cells with epithelial cell characteristics to undergo EMT in a concentration-dependent manner. Following TGF-beta1 treatment, A549 cells induced EMT characterized by cells morphological changes, loss of epithelial markers Ecaherin and cytokeratin, increased stress fiber reorganization by F-actin, and cytokeratin replacement by vimentin. Although IL-1beta failed to induce A549 cells to undergo EMT, the combination of TGF-beta1 and IL-1beta showed synergy effects in cells morphology changes and the expression of mesenchymal markers. The snail expression study using RT-PCR analysis provided that loss of E-cadherin expression was associated with snail expression. Stimulation of A54 cells with TGF-beta1 plus IL-1beta revealed a higher level of snail expression. Our data showed that EMT of A549 cells might be closely associated with snail expression.
Keyword
MeSH Terms
-
Actins/metabolism
Cadherins/metabolism
Cell Differentiation
Cell Line, Tumor
Dose-Response Relationship, Drug
Epithelium/*metabolism
Fluorescent Antibody Technique, Indirect
*Gene Expression Regulation, Neoplastic
Humans
Keratins/metabolism
Mesoderm/*metabolism
Microscopy, Fluorescence
Reverse Transcriptase Polymerase Chain Reaction
Transcription, Genetic
Transforming Growth Factor beta1/metabolism/*physiology
Vimentin/metabolism
Figure
Reference
-
1. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004. 350:2645–2653.
Article2. Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001. 134:136–151.
Article3. Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003. 12:25–29.
Article4. Nicolas FJ, Lehmann K, Warne PH, Hill CS, Downward J. Epithelial to mesenchymal transition in Madin-Darby canine kidney cells is accompanied by down-regulation of Smad3 expression, leading to resistance to transforming growth factor-beta-induced growth arrest. J Biol Chem. 2003. 278:3251–3256.5. Do JY. Epithelial to mesenchymal transition in CAPD patients. Yeungnam Univ J Med. 2006. 23:10–18.
Article6. Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995. 26:678–690.
Article7. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001. 56:907–915.8. Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001. 163:1476–1483.9. Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005. 6:56.
Article10. Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004. 76:29–37.11. Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gobe G, Johnson DW. Interleukin-1beta induces human proximal tubule cell injury, alpha-smooth muscle actin expression and fibronectin production. Kidney Int. 2002. 62:31–40.12. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002. 2:442–454.
Article13. Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995. 154:8–20.
Article14. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002. 110:341–350.
Article15. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003. 9:964–968.16. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995. 130:393–405.
Article17. Okada H, Inoue T, Kanno Y, Kobayashi T, Ban S, Kalluri R, Suzuki H. Renal fibroblast-like cells in Goodpasture syndrome rats. Kidney Int. 2001. 60:597–606.
Article18. Zeisberg M, Maeshima Y, Mosterman B, Kalluri R. Renal fibrosis. Extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. Am J Pathol. 2002. 160:2001–2008.19. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005. 166:1321–1332.20. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997. 390:465–471.21. Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA. 2001. 98:6686–6691.22. Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000. 1:91–100.
Article23. Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001. 105:425–431.
Article24. Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000. 2:76–83.
Article25. Potter E, Bergwitz C, Brabant G. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocr Rev. 1999. 20:207–239.
Article26. Kim K, Lu Z, Hay ED. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002. 26:463–476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardamonin Suppresses TGF-beta1-Induced Epithelial Mesenchymal Transition via Restoring Protein Phosphatase 2A Expression
- Epithelial-Mesenchymal Transitions of Bile Duct Epithelial Cells in Primary Hepatolithiasis
- Apolipoprotein A1 Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition of Alveolar Epithelial Cells
- Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells
- Epithelial to Mesenchymal Transition of Mesothelial Cells in Tuberculous Pleurisy