J Korean Med Sci.
2007 Apr;22(2):177-182. 10.3346/jkms.2007.22.2.177.
Marked Suppression of Ghrelin Concentration by Insulin in Prader-Willi Syndrome
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jindk@smc.samsung.co.kr
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Clinical Research Center, Samsung Biomedical Research Institute, Seoul, Korea.
- 5Department of Pediatrics, College of Medicine, Inha University, Incheon, Korea.
- KMID: 1713157
- DOI: http://doi.org/10.3346/jkms.2007.22.2.177
Abstract
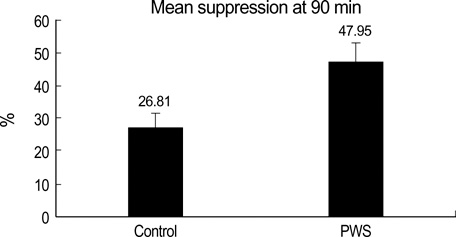
- The plasma ghrelin has been reported to be elevated in Prader-Willi syndrome (PWS) and modulated by insulin. It was hypothesized that insulin might have a more pronounced effect on reducing plasma ghrelin in PWS patients, which would influence appetite. This study investigated the degree of ghrelin suppression using an euglycemic hyperinsulinemic clamp in children with PWS (n=6) and normal children (n=6). After a 90-min infusion of insulin, the plasma ghrelin level decreased from a basal value of 0.86+/-0.15 to 0.58+/-0.12 ng/mL in the controls, and from 2.38+/-0.76 to 1.12+/-0.29 ng/mL in children with PWS (p=0.011). The area under the curve below the baseline level over the 90 min insulin infusion was larger in children with PWS than in controls (-92.82+/-44.4 vs. -10.41+/-2.87 ng/mL/90 min) (p=0.011). The insulin sensitivity measured as the glucose infusion rate at steady state was similar in the two groups (p=0.088). The decrease in the ghrelin levels in response to insulin was more pronounced in the children with PWS than in the controls. However, the level of ghrelin was always higher in the children with PWS during the clamp study. This suggests that even though insulin sensitivity to ghrelin is well maintained, an increase in the baseline ghrelin levels is characteristic of PWS.
Keyword
MeSH Terms
Figure
Reference
-
1. Ishikawa T, Kibe T, Wada Y. Deletion of small nuclear ribonucleoprotein polypeptide N (SNRPN) in Prader-Willi syndrome detected by fluorescence in situ hybridization: two sibs with the typical phenotype without a cytogenetic deletion in chromosome 15q. Am J Med Genet. 1996. 62:350–352.
Article2. MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997. 6:1873–1878.
Article3. Prader A, Labhart A, Willi H. Ein Syndrom von Adipositas, Kleinwuchs, Kryptorchismus und Oligophrenie nach Myatonieartigem Zustand im Neugeborenenalter. Schweiz Med Wochenschr. 1956. 86:1260–1261.4. Butler MG, Meaney FJ. An anthropometric study of 38 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1987. 26:445–455.
Article5. Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol. 1987. 29:145–152.
Article6. Curfs LM, Verhulst FC, Fryns JP. Behavioral and emotional problems in youngsters with Prader-Willi syndrome. Genet Couns. 1991. 2:33–41.7. Lindgren AC, Barkeling B, Hagg A, Ritzen EM, Marcus C, Rossner S. Eating behavior in Prader-Willi syndrome, normal weight, and obese control groups. J Pediatr. 2000. 137:50–55.
Article8. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999. 402:656–660.
Article9. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001. 409:194–198.
Article10. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000. 407:908–913.
Article11. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001. 86:5992.
Article12. Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001. 50:707–709.
Article13. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002. 87:240–244.
Article14. Jo DS, Lee JU, Kim SY, Kang CW, Hwang PH, Lee DY. Plasma ghrelin levels and its relationship with obesity in obese children. J Korean Soc Pediatr Endocrinol. 2004. 9:179–185.15. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001. 86:4753–4758.
Article16. Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001. 24:RC19–RC21.
Article17. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001. 50:1714–1719.
Article18. Jin DK. Ghrelin in the Prader-Willi Syndrome. J Korean Soc Pediatr Endocrinol. 2003. 8:119–125.19. Paik KH, Jin DK, Song SY, Lee JE, Ko SH, Song SM, Kim JS, Oh YJ, Kim SW, Lee SH, Kim SH, Kwon EK, Choe YH. Correlation between fasting plasma ghrelin levels and age, body mass index (BMI), BMI percentiles, and 24-hour plasma ghrelin profiles in Prader-Willi syndrome. J Clin Endocrinol Metab. 2004. 89:3885–3889.
Article20. Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002. 87:3997–4000.
Article21. Mohlig M, Spranger J, Otto B, Ristow M, Tschop M, Pfeiffer AF. Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J Endocrinol Invest. 2002. 25:RC36–RC38.
Article22. Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003. 284:E313–E316.
Article23. Schaller G, Schmidt A, Pleiner J, Woloszczuk W, Wolzt M, Luger A. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes. 2003. 52:16–20.
Article24. Goldstone AP, Thomas EL, Brynes AE, Castroman G, Edwards R, Ghatei MA, Frost G, Holland AJ, Grossman AB, Korbonits M, Bloom SR, Bell JD. Elevated fasting plasma ghrelin in prader-willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. J Clin Endocrinol Metab. 2004. 89:1718–1726.
Article25. Choe YH, Song SY, Paik KH, Oh YJ, Chu SH, Yeo SH, Kwon EK, Kim EM, Rha MY, Jin DK. Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader-Willi syndrome. J Clin Endocrinol Metab. 2005. 90:5441–5445.
Article26. Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003. 52:2923–2927.
Article27. Kim SY, Shin JY, Jung MJ, Choi BM, Lee JH, Lee KH. Relationship between serum ghrelin and insulin resistance in obese children and adolescents. J Korean Soc Pediatr Endocrinol. 2005. 10:211–217.28. Caixas A, Bashore C, Nash W, Pi-Sunyer F, Laferrere B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. J Clin Endocrinol Metab. 2002. 87:1902.
Article29. Broglio F, Koetsveld Pv P, Benso A, Gottero C, Prodam F, Papotti M, Muccioli G, Gauna C, Hofland L, Deghenghi R, Arvat E, Van Der Lely AJ, Ghigo E. Ghrelin secretion is inhibited by either somatostatin or cortistatin in humans. J Clin Endocrinol Metab. 2002. 87:4829–4832.
Article30. Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003. 144:916–921.
Article31. Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, Maccario M, Deghenghi R, van der Ley AJ, Ghigo E. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab. 2003. 88:4268–4272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ghrelin in the Prader-Willi Syndrome
- A Case of Prader Willi syndrome
- One Case of the Prader-Willi Syndrome
- A Case of Prader-Willi Syndrome with Diabetes Mellitus
- Peptide YY, Cholecystokinin, Insulin and Ghrelin Response to Meal did not Change, but Mean Serum Levels of Insulin is Reduced in Children with Prader-Willi Syndrome