J Korean Med Sci.
2005 Aug;20(4):566-572. 10.3346/jkms.2005.20.4.566.
Murine Model of Buckwheat Allergy by Intragastric Sensitization with Fresh Buckwheat Flour Extract
- Affiliations
-
- 1Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea. jsjs87@ajou.ac.kr
- 2Laboratory of Immunology, Institute for Medical Science, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Pediatrics and Allergy Research Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1712734
- DOI: http://doi.org/10.3346/jkms.2005.20.4.566
Abstract
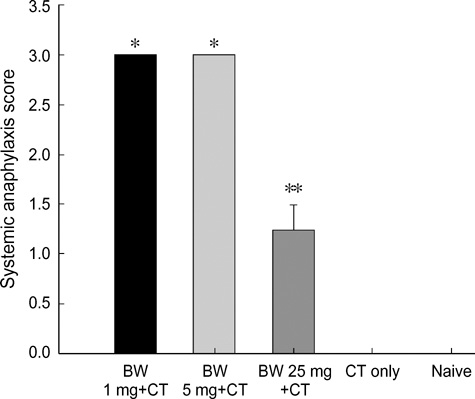
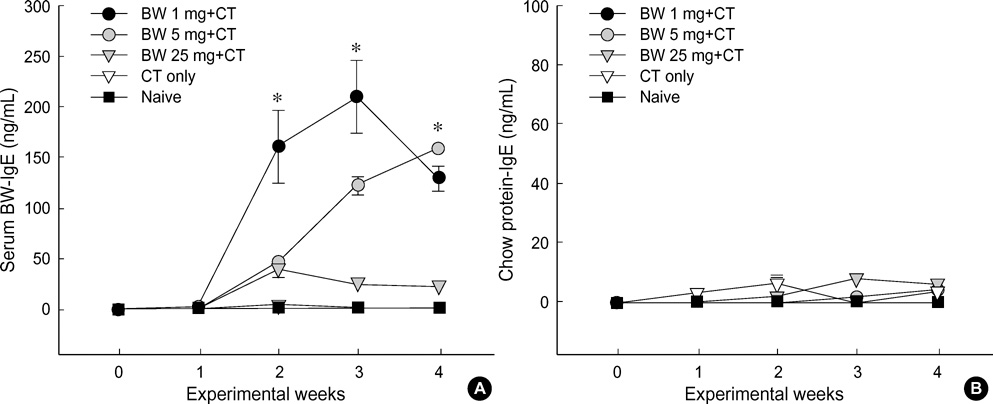
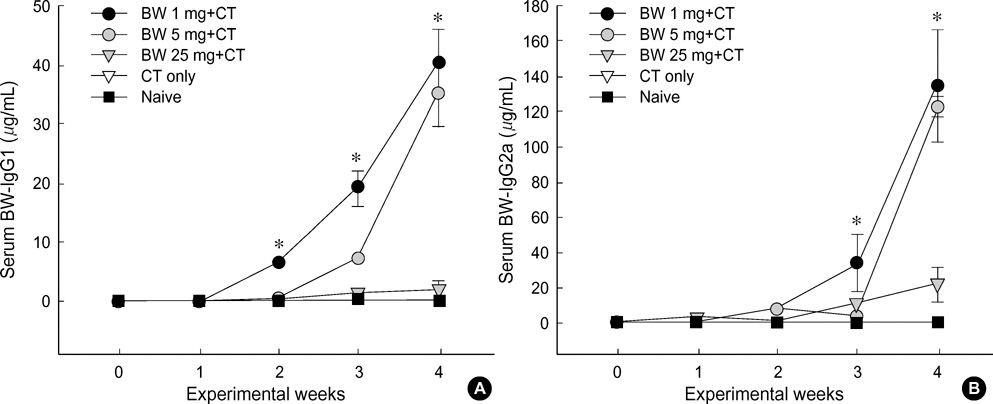
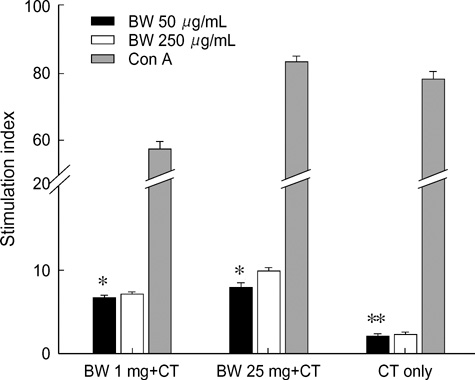
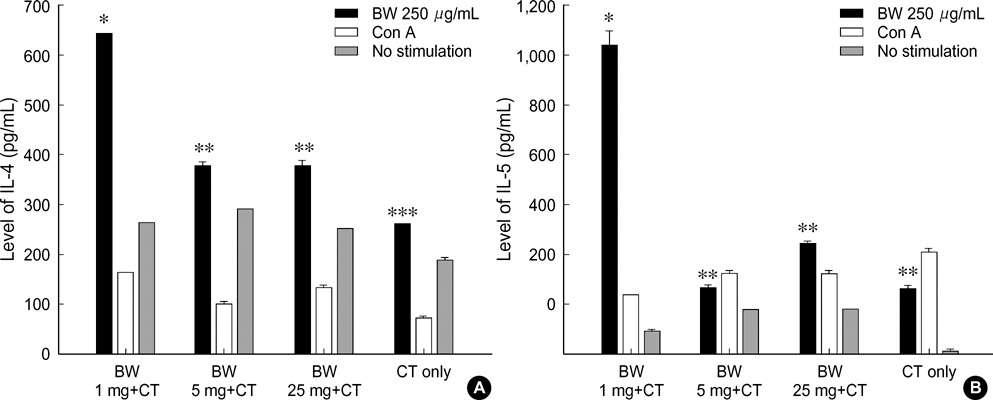
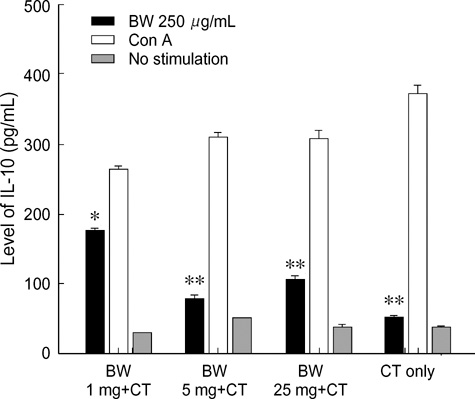
- Food allergies affect about 4% of the Korean population, and buckwheat allergy is one of the most severe food allergies in Korea. The purpose of the present study was to develop a murine model of IgE-mediated buckwheat hypersensitivity induced by intragastric sensitization. Young female C3H/HeJ mice were sensitized and challenged intragastricly with fresh buckwheat flour (1, 5, 25 mg/dose of proteins) mixed in cholera toxin, followed by intragastric challenge. Anaphylactic reactions, antigen-specific antibodies, splenocytes proliferation assays and cytokine productions were evaluated. Oral buckwheat challenges of sensitized mice provoked anaphylactic reactions such as severe scratch, perioral/periorbital swellings, or decreased activity. Reactions were associated with elevated levels of buckwheatspecific IgE antibodies. Splenocytes from buckwheat allergic mice exhibited significantly greater proliferative responses to buckwheat than non-allergic mice. Buckwheat-stimulated IL-4, IL-5, and INF-gamma productions were associated with elevated levels of buckwheat-specific IgE in sensitized mice. In this model, 1 mg and 5 mg dose of sensitization produced almost the same degree of Th2-directed immune response, however, a 25 mg dose showed blunted antibody responses. In conclusion, we developed IgE-mediated buckwheat allergy by intragastric sensitization and challenge, and this model could provide a good tool for future studies.
Keyword
MeSH Terms
-
Anaphylaxis/blood/immunology
Animals
Cell Proliferation/drug effects
Comparative Study
Disease Models, Animal
Dose-Response Relationship, Drug
Enzyme-Linked Immunosorbent Assay
Fagopyrum/*immunology
Female
*Flour
Food Hypersensitivity/blood/*immunology
Immunoglobulin E/blood/immunology
Immunoglobulin G/blood/immunology
Interferon Type II/biosynthesis
Interleukin-4/biosynthesis
Interleukin-5/biosynthesis
Mice
Mice, Inbred C3H
Plant Extracts/administration & dosage/immunology
Research Support, Non-U.S. Gov't
Spleen/cytology/drug effects/metabolism
Stomach/drug effects/*immunology
T-Lymphocytes/cytology/drug effects/metabolism
Time Factors
Figure
Cited by 1 articles
-
Effect of Cosensitization with Buckwheat Flour Extract on the Production of House Dust Mite-specific IgE
Youn Ho Shin, Myung Hyun Sohn, Sejo Oh, Kyung Eun Lee, Tae Soon Yong, Jung Won Park, Chein Soo Hong, Kyu-Earn Kim, Soo-Young Lee
J Korean Med Sci. 2007;22(2):198-204. doi: 10.3346/jkms.2007.22.2.198.
Reference
-
1. Wieslander G. Review on buckwheat allergy. Allergy. 1996. 51:661–665.
Article2. Smith HL. Buckwheat-poisoning with report of a case in man. Allergy Proc. 1990. 11:193–196.3. Horesh AJ. Buckwheat sensitivity in children. Ann Allergy. 1972. 30:685–689.4. Davidson AE, Passero MA, Settipane GA. Buckwheat induced anaphylaxis: a case report. Ann Allergy. 1992. 69:439–440.5. Nakamura S. On occupational allergic asthma of different kinds newly found in our allergic clinic. J Asthma Res. 1972. 10:37–42.6. Gohte CJ, Wieslander G, Ancker K, Forsbeck M. Buckwheat allergy: health food, an inhalation health risk. Allergy. 1983. 38:155–159.
Article7. Valdivieso R, Moneo I, Pola J, Munoz T, Zapata C, Hinojosa M, Losada E. Occupational asthma and contact urticaria caused by buckwheat flour. Ann Allergy. 1989. 63:149–152.8. Matsumura T, Tateno K, Yugami S, Kuroume T. Six cases of buckwheat asthma induced by buckwheat flour attached to buckwheat chaff in pillows. J Asthma Res. 1964. 15:219–227.
Article9. Lee KY, Kim KE. A study on the method of exclusion of unnecessary allergens from the vaccines for immunotherapy. J Korean Soc Allergol. 1988. 8:150–164.10. Kwon YB, Lee KY. Two cases of children with buckwheat allergy confirmed by oral challenge test. J Korean Pediatr Soc. 1985. 28:82–87.11. Lee SY, Lee KS, Hong CH, Lee KY. Three cases of childhood nocturnal asthma due to buckwheat allergy. Allergy. 2001. 56:763–766.
Article12. Park HS, Nahm DH. Buckwheat flour hypersensitivity: an occupational asthma in a noodle maker. Clin Exp Allergy. 1996. 26:423–427.
Article13. Park JW, Kang DB, Kim CW, Ko SH, Yum HY, Kim KE, Hong CS, Lee KY. Identification and characterization of the major allergens of buckwheat. Allergy. 2000. 55:1035–1041.
Article14. Kondo Y, Urisu A, Wada E, Tsuruta M, Yasaki T, Yamada K, Masuda S, Morita Y. Allergen analysis of buckwheat by the immunoblotting method. Arerugi. 1993. 42:142–148.15. Schiffner R, Przybilla B, Burgdorff T, Landthaler M, Stolz W. Anaphylaxis to buckwheat. Allergy. 2001. 56:1020–1021.
Article16. Ito K, Inagaki-Ohara K, Murosaki S, Nishimura H, Shimokata T, Torii S, Matsuda T, Yoshikai Y. Murine model of IgE production with a predominant Th2-response by feeding protein antigen without adjuvants. Eur J Immunol. 1997. 27:3427–3437.
Article17. Kiyono H, McGhee JR, Wannemuehler MJ, Michalek SM. Lack of oral tolerance in C3H/HeJ mice. J Exp Med. 1982. 155:605–610.
Article18. Hanson DG. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J Immunol. 1981. 127:1518–1524.19. Strobel S, Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984. 18:588–594.
Article20. Strobel S. Neonatal oral tolerance. Ann NY Acad Sci. 1996. 778:88–102.21. Mowat AM. The regulation of immune responses to dietary protein antigens. Immunology Today. 1987. 8:93–98.
Article22. Lamont AG, Mowat AM, Parrott DM. Priming of systemic and local delayed-type hypersensitivity responses by feeding low doses of ovalbumin to mice. Immunology. 1989. 66:595–599.23. Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, Stanley JS, Burks AW, Bannon GA, Sampson HA. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000. 106:150–158.
Article24. Institute of Laboratory Animal Resources Commission of Life Sciences NRC. Guide for the Care and Use of Laboratory Animals. 1996. National Academy Press.25. Lee KY, Lee SY, Jeoung BJ, Kim KE, Kim DS. Cross-reactivity among four cereals: rice, barley, wheat flour and buckwheat flour. J Korean Soc Allergol. 1993. 13:1–10.26. Sampson HA. Food allergy. JAMA. 1997. 278:1888–1894.
Article27. Rance F, Abbal M, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol. 2002. 109:1027–1033.28. Knippels LM, van der Kleij HP, Koppelman SJ, Houben GF, Penninks AH. Comparison of antibody responses to hen's egg and cow's milk proteins in orally sensitized rats and food-allergic patients. Allergy. 2000. 55:251–258.
Article29. Helm RM, Furuta GT, Stanley JS, Ye J, Cockrell G, Connaughton C, Simpson P, Bannon GA, Burks AW. A neonatal swine model for peanut allergy. J Allergy Clin Immunol. 2002. 109:136–142.
Article30. Abouzied MM, Azcona JI, Braselton WE, Pestka JJ. Immunochemical assessment of Mycotoxins in 1989 grain foods: evidence for deoxynivalenol (vomitoxin) contamination. Appl Environ Microbiol. 1991. 57:672–677.
Article31. Zhou HR, Yan D, Pestka JJ. Induction of cytokine gene expression in mice after repeated and subchronic oral exposure to Vomitoxin (Deoxynivalenol): differential toxin-induced hyporesponsiveness and recovery. Toxicol Appl Pharmacol. 1998. 151:347–358.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparative Study on Allergenicity of Raw and Hypoallergenic Buckwheat flour
- Dermatophagoides Farinae, an Important Allergenic Substance in Buckwheat-Husk Pillows
- A Study on the Cross-allergenicity between Buckwheat and Rice Flour Using IgE-immunoblot Inhibition and ELISA-inhibition Test
- One Case of Buckwheat Allergy Proved by Oral Provacation Test
- Three Cases of Buckwheat Allergy