J Vet Sci.
2013 Dec;14(4):405-412. 10.4142/jvs.2013.14.4.405.
Inhibitory effects of osteoprotegerin on osteoclast formation and function under serum-free conditions
- Affiliations
-
- 1College of Veterinary Medicine, Yangzhou University, and Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, China. liuzongping@yzu.edu.cn
- KMID: 1712306
- DOI: http://doi.org/10.4142/jvs.2013.14.4.405
Abstract
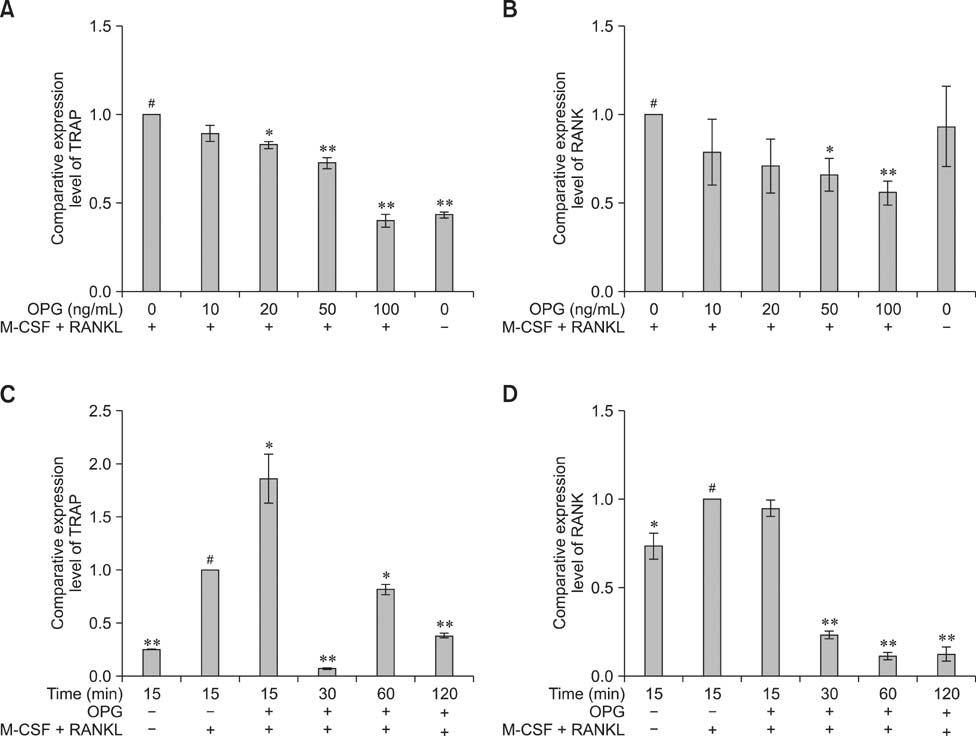
- The purpose of this study was to determine whether osteoprotegerin (OPG) could affect osteoclat differentiation and activation under serum-free conditions. Both duck embryo bone marrow cells and RAW264.7 cells were incubated with macrophage colony stimulatory factor (M-CSF) and receptor activator for nuclear factor kappaB ligand (RANKL) in serum-free medium to promote osteoclastogenesis. During cultivation, 0, 10, 20, 50, and 100 ng/mL OPG were added to various groups of cells. Osteoclast differentiation and activation were monitored via tartrate-resistant acid phosphatase (TRAP) staining, filamentous-actin rings analysis, and a bone resorption assay. Furthermore, the expression osteoclast-related genes, such as TRAP and receptor activator for nuclear factor kappaB (RANK), that was influenced by OPG in RAW264.7 cells was examined using real-time polymerase chain reaction. In summary, findings from the present study suggested that M-CSF with RANKL can promote osteoclast differentiation and activation, and enhance the expression of TRAP and RANK mRNA in osteoclasts. In contrast, OPG inhibited these activities under serum-free conditions.
Keyword
MeSH Terms
-
Acid Phosphatase/genetics/metabolism
Animals
Avian Proteins/*pharmacology
Bone Marrow Cells/drug effects/*metabolism
Cells, Cultured
Ducks
Embryo, Nonmammalian/drug effects/metabolism
Isoenzymes/genetics/metabolism
Macrophage Colony-Stimulating Factor/metabolism
Osteoclasts/cytology/*drug effects/*metabolism
Osteoprotegerin/*pharmacology
RANK Ligand/metabolism
Real-Time Polymerase Chain Reaction
Receptor Activator of Nuclear Factor-kappa B/genetics/metabolism
Acid Phosphatase
Avian Proteins
Isoenzymes
Macrophage Colony-Stimulating Factor
Osteoprotegerin
RANK Ligand
Receptor Activator of Nuclear Factor-kappa B
Figure
Reference
-
1. Aeschlimann D, Evans BAJ. The vital osteoclast: how is it regulated? Cell Death Differ. 2004; 11:S5–S7.
Article2. Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr. 2009; 19:171–180.
Article3. Boyce BF, Yao Z, Zhang Q, Guo R, Lu Y, Schwarz EM, Xing L. New roles for osteoclasts in bone. Ann N Y Acad Sci. 2007; 1116:245–254.
Article4. Bridgham JT, Johnson AL. Characterization of chicken TNFR superfamily decoy receptors, DcR3 and osteoprotegerin. Biochem Biophys Res Commun. 2003; 307:956–961.
Article5. Chamoux E, Houde N, L'Eriger K, Roux S. Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway. J Cell Physiol. 2008; 216:536–542.
Article6. Chen J, He JQ, Zhen SY, Huang LQ. OPG inhibits gene expression of RANK and CAII in mouse osteoclast-like cell. Rheumatol Int. 2012; 32:3993–3998.
Article7. Chen X, Zhu G, Jin T, Gu S, Xiao H, Qiu J. Cadmium induces differentiation of RAW264.7 cells into osteoclasts in the presence of RANKL. Food Chem Toxicol. 2011; 49:2392–2397.
Article8. Choi HJ, Park YR, Nepal M, Choi BY, Cho NP, Choi SH, Heo SR, Kim HS, Yang MS, Soh Y. Inhibition of osteoclastogenic differentiation by Ikarisoside A in RAW 264.7 cells via JNK and NF-κB signaling pathways. Eur J Pharmacol. 2010; 636:28–35.
Article9. Cuetara BLV, Crotti TN, O'Donoghue AJ, McHugh KP. Cloning and characterization of osteoclast precursors from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim. 2006; 42:182–188.
Article10. Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005; 350:1–13.
Article11. Fu YX, Gu JH, Wang ST, Wang Y, Zhao HY, Liu W, Tong XS, Liu ZP. Characteristics comparison of osteoclasts induced by M-CSF and RANKL. Chinese J Vet Sci. 2013; 33:94–97.12. Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, Hakozaki A, Uchikawa S, Matsumoto M, Chiba K, Kimura T, Okada Y, Matsuo K, Yoshida H, Toyama Y. IL-27 abrogates receptor activator of NF-κB ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009; 183:2397–2406.
Article13. Gay CV. Avian osteoclasts. Calcif Tissue Int. 1991; 49:153–154.
Article14. Gray AW, Davies ME, Jeffcott LB. Generation and activity of equine osteoclasts in vitro: effects of the bisphosphonate pamidronate (APD). Res Vet Sci. 2002; 72:105–113.
Article15. Grigoriadis AE, Kennedy M, Bozec A, Brunton F, Stenbeck G, Park IH, Wagner EF, Keller GM. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010; 115:2769–2776.
Article16. Gu JH, Liu JD, Shen Y, Liu ZP. Effects of RANKL, osteoprotegerin, calcium and phosphorus on survival and activation of Muscovy duck osteoclasts in vitro. Vet J. 2009; 181:321–325.
Article17. Hakeda Y, Kobayashi Y, Yamaguchi K, Yasuda H, Tsuda E, Higashio K, Miyata T, Kumegawa M. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem Biophys Res Commun. 1998; 251:796–801.
Article18. Henriksen K, Leeming DJ, Byrjalsen I, Nielsen RH, Sorensen MG, Dziegiel MH, Martin TJ, Christiansen C, Qvist P, Karsdal MA. Osteoclasts prefer aged bone. Osteoporos Int. 2007; 18:751–759.
Article19. Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer. 2001; 92:460–470.
Article20. Hou L, Hou J, Yao J, Zhou ZL. Effects of osteoprotegerin from transfection of pcDNA3.1(+)/chOPG on bioactivity of chicken osteoclasts. Acta Vet Scand. 2011; 53:21.
Article21. Itonaga I, Sabokbar A, Sun SG, Kudo O, Danks L, Ferguson D, Fujikawa Y, Athanasou NA. Transforming growth factor-β induces osteoclast formation in the absence of RANKL. Bone. 2004; 34:57–64.
Article22. Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009; 19:61–72.
Article23. Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.
Article24. Lee JW, Kobayashi Y, Nakamichi Y, Udagawa N, Takahashi N, Im NK, Seo HJ, Jeon WB, Yonezawa T, Cha BY, Woo JT. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem Pharmacol. 2010; 80:352–361.
Article25. Lee SK, Goldring SR, Lorenzo JA. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995; 136:4572–4581.
Article26. Lemay S, Lebedeva TV, Singh AK. Inhibition of cytokine gene expression by sodium salicylate in a macrophage cell line through an NF-κB-independent mechanism. Clin Diagn Lab Immunol. 1999; 6:567–572.
Article27. Liu H, Zhang R, Ko SY, Oyajobi BO, Papasian CJ, Deng HW, Zhang S, Zhao M. Microtubule assembly affects bone mass by regulating both osteoblast and osteoclast functions: stathmin deficiency produces an osteopenic phenotype in mice. J Bone Miner Res. 2011; 26:2052–2067.
Article28. Nakamura I, Takahashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012; 22:167–177.
Article29. Nakayama T, Mizoguchi T, Uehara S, Yamashita T, Kawahara I, Kobayashi Y, Moriyama Y, Kurihara S, Sahara N, Ozawa H, Udagawa N, Takahashi N. Polarized osteoclasts put marks of tartrate-resistant acid phosphatase on dentin slices-a simple method for identifying polarized osteoclasts. Bone. 2011; 49:1331–1339.
Article30. Oguro A, Kawase T, Orikasa M. NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway but not the monocytic or preosteoclastic pathway in vitro. In vitro Cell Dev Biol Anim. 2003; 39:243–248.
Article31. Price CP, Kirwan A, Vader C. Tartrate-resistant acid phosphatase as a marker of bone resorption. Clin Chem. 1995; 41:641–643.
Article32. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997; 89:309–319.
Article33. Tang CH, Chang CS, Tan TW, Liu SC, Liu JF. The novel isoflavone derivatives inhibit RANKL-induced osteoclast formation. Eur J Pharmacol. 2010; 648:59–66.
Article34. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289:1504–1508.
Article35. Vincent C, Kogawa M, Findlay DM, Atkins GJ. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J Bone Miner Metab. 2009; 27:114–119.
Article36. Wang Y, Hou JF, Zhou ZL. Chicken receptor activator of nuclear factor-κB ligand induces formation of chicken osteoclasts from bone marrow cells and also directly activates mature osteoclasts. Poult Sci. 2008; 87:2344–2349.
Article37. Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009; 2:56–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coronary artery calcification and serum markers
- Inhibitory action of bisphosphonates on bone resorption does not involve the regulation of RANKL and OPG expression
- Expression of Osteoprotegerin and Osteoclast Differentiation Factor in Human Periodontal Ligament Fibroblast Cells
- Involvement of the Ca2+ signaling pathway in osteoprotegerin inhibition of osteoclast differentiation and maturation
- Attenuation of RANKL-induced Osteoclast Formation via p38-mediated NFATc1 Signaling Pathways by Extract of Euphorbia Lathyris L