Korean J Radiol.
2013 Dec;14(6):923-930. 10.3348/kjr.2013.14.6.923.
Rat Model of Hindlimb Ischemia Induced via Embolization with Polyvinyl Alcohol and N-Butyl Cyanoacrylate
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 110-744, Korea. angiointervention@gmail.com
- 2Institute of Radiation Medicine, Seoul National University Medical Research Center (SNUMRC), Seoul 110-799, Korea.
- 3Department of Pathology, Seoul National University Hospital, Seoul 110-744, Korea.
- KMID: 1711459
- DOI: http://doi.org/10.3348/kjr.2013.14.6.923
Abstract
OBJECTIVE
To investigate the feasibility of a rat model on hindlimb ischemia induced by embolization from the administration of polyvinyl alcohol (PVA) particles or N-butyl cyanoacrylate (NBCA).
MATERIALS AND METHODS
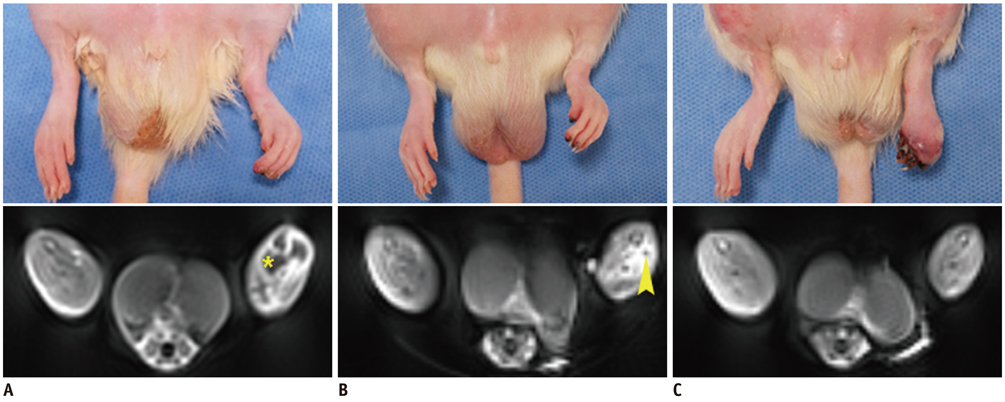
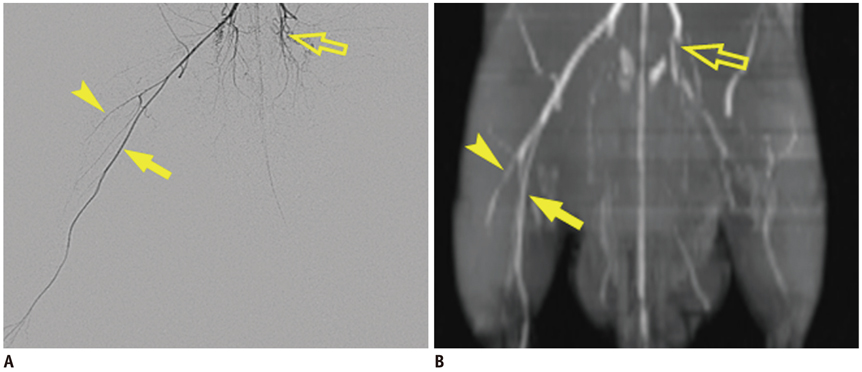
Unilateral hindlimb ischemia was induced by embolization with NBCA (n = 4), PVA (n = 4) or surgical excision (n = 4) in a total of 12 Sprague-Dawley rats. On days 0, 7 and 14, the time-of-flight magnetic resonance angiography (TOF-MRA) and enhanced MRI were obtained as scheduled by using a 3T-MR scanner. The clinical ischemic index, volume change and degree of muscle necrosis observed on the enhanced MRI in the ischemic hindlimb were being compared among three groups using the analysis of variance. Vascular patency on TOF-MRA was evaluated and correlated with angiographic findings when using an inter-rater agreement test.
RESULTS
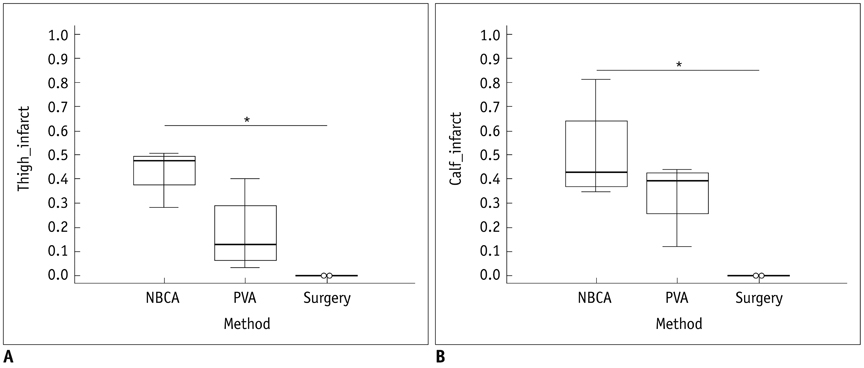
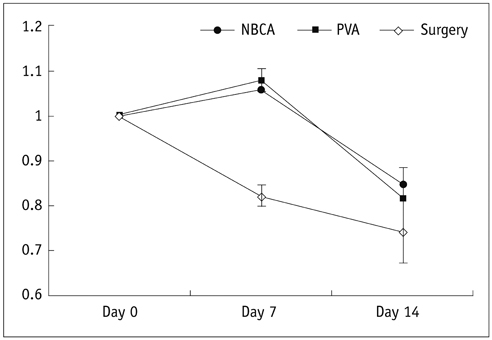
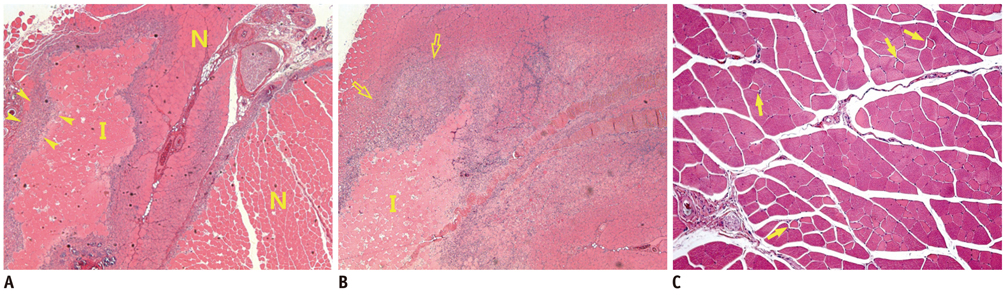
There was a technical success rate of 100% for both the embolization and surgery groups. The clinical ischemic index did not significantly differ. On day 7, the ratios of the muscular infarctions were 0.436, 0.173 and 0 at thigh levels and 0.503, 0.337 and 0 at calf levels for the NBCA, PVA and surgery groups, respectively. In addition, the embolization group presented increased volume and then decreased volume on days 7 and 14, respectively. The surgery group presented a gradual volume decrease. Good correlation was shown between the TOF-MRA and angiographic findings (kappa value of 0.795).
CONCLUSION
The examined hindlimb ischemia model using embolization with NBCA and PVA particles in rats is a feasible model for further research, and muscle necrosis was evident as compared with the surgical model.
Keyword
MeSH Terms
-
Animals
*Disease Models, Animal
Embolization, Therapeutic/*adverse effects
Enbucrilate/administration & dosage/*toxicity
Feasibility Studies
Hindlimb/*blood supply
Injections, Intra-Arterial
Ischemia/*chemically induced/diagnosis
Magnetic Resonance Angiography/*methods
Male
Polyvinyl Alcohol/administration & dosage/*toxicity
Rats
Rats, Sprague-Dawley
Tissue Adhesives/administration & dosage/toxicity
Enbucrilate
Polyvinyl Alcohol
Tissue Adhesives
Figure
Reference
-
1. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008; 89:422–429.2. Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit? Circulation. 2006; 114:861–866.3. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463–e654.4. Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004; 287:C572–C579.5. Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003; 10:83–97.6. Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006; 10:45–55.7. Menasché P. Cell therapy for peripheral arterial disease. Curr Opin Mol Ther. 2010; 12:538–545.8. Mughal NA, Russell DA, Ponnambalam S, Homer-Vanniasinkam S. Gene therapy in the treatment of peripheral arterial disease. Br J Surg. 2012; 99:6–15.9. Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003; 2:863–871.10. Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. 2001; 125:67–71.11. Zhuang ZW, Shi J, Rhodes JM, Tsapakos MJ, Simons M. Challenging the surgical rodent hindlimb ischemia model with the miniinterventional technique. J Vasc Interv Radiol. 2011; 22:1437–1446.12. Hsu LY, Wragg A, Anderson SA, Balaban RS, Boehm M, Arai AE. Automatic assessment of dynamic contrast-enhanced MRI in an ischemic rat hindlimb model: an exploratory study of transplanted multipotent progenitor cells. NMR Biomed. 2008; 21:111–119.13. Sbarbati A, Marzola P, Nicolato E, Farace P, Asperio RM, Lunati E, et al. Dynamic MRI reveals that the magnitude of the ischemia-related enhancement in skeletal muscle is age-dependent. Magn Reson Med. 2003; 49:386–390.14. Stekelenburg A, Strijkers GJ, Parusel H, Bader DL, Nicolay K, Oomens CW. Role of ischemia and deformation in the onset of compression-induced deep tissue injury: MRI-based studies in a rat model. J Appl Physiol (1985). 2007; 102:2002–2011.15. Delli Pizzi S, Madonna R, Caulo M, Romani GL, De Caterina R, Tartaro A. MR angiography, MR imaging and proton MR spectroscopy in-vivo assessment of skeletal muscle ischemia in diabetic rats. PLoS One. 2012; 7:e44752.16. Jaspers K, Versluis B, Leiner T, Dijkstra P, Oostendorp M, van Golde JM, et al. MR angiography of collateral arteries in a hind limb ischemia model: comparison between blood pool agent Gadomer and small contrast agent Gd-DTPA. PLoS One. 2011; 6:e16159.17. Paek R, Chang DS, Brevetti LS, Rollins MD, Brady S, Ursell PC, et al. Correlation of a simple direct measurement of muscle pO(2) to a clinical ischemia index and histology in a rat model of chronic severe hindlimb ischemia. J Vasc Surg. 2002; 36:172–179.18. Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol. 2002; 283:H2012–H2020.19. Takasawa C, Seiji K, Matsunaga K, Matsuhashi T, Ohta M, Shida S, et al. Properties of N-butyl cyanoacrylate-iodized oil mixtures for arterial embolization: in vitro and in vivo experiments. J Vasc Interv Radiol. 2012; 23:1215–1221.20. Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005; 41:312–320.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple Cerebral Infarction after Injection of N-Butyl-2-Cyanoacrylate for Gastric Variceal Bleeding
- Is glue embolization safe and effective for gastrointestinal bleeding?
- Guidewire-Induced Perforation of Distal Circumflex Artery Treated with Transcatheter Embolization of Polyvinyl Alcohol Form
- Interventional Treatment of Bleeding
- Percutaneous N-Butyl Cyanoacrylate Embolization of a Pancreatic Pseudoaneurysm after Failed Attempts of Transcatheter Embolization