Lab Anim Res.
2014 Jun;30(2):64-72. 10.5625/lar.2014.30.2.64.
Establishment of linear accelerator-based image guided radiotherapy for orthotopic 4T1 mouse mammary tumor model
- Affiliations
-
- 1Department of Radiation Oncology, Gachon University Gil Medical Center, Incheon, Korea. kyu22@gilhospital.com
- 2Laboratory of Tumor Suppressor, Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, Korea.
- KMID: 1707445
- DOI: http://doi.org/10.5625/lar.2014.30.2.64
Abstract
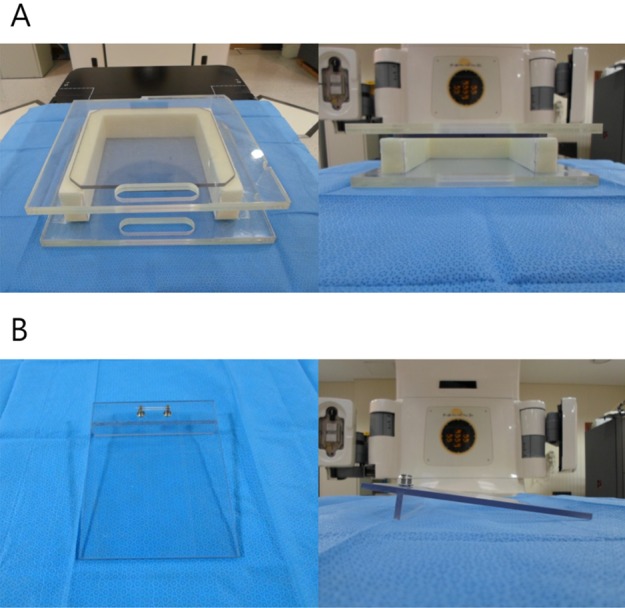
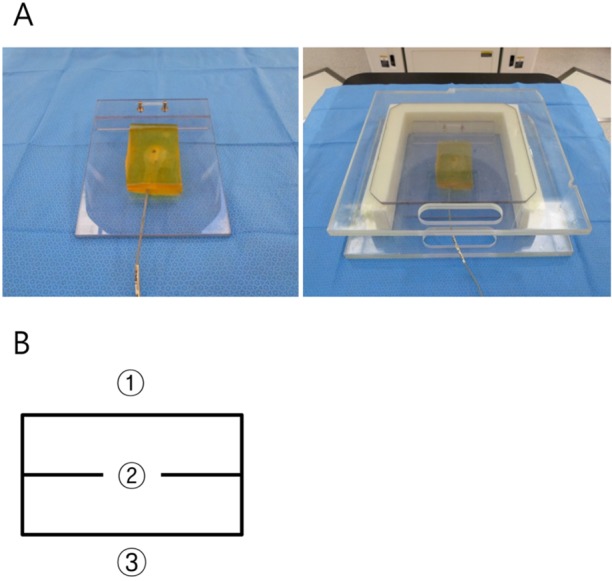
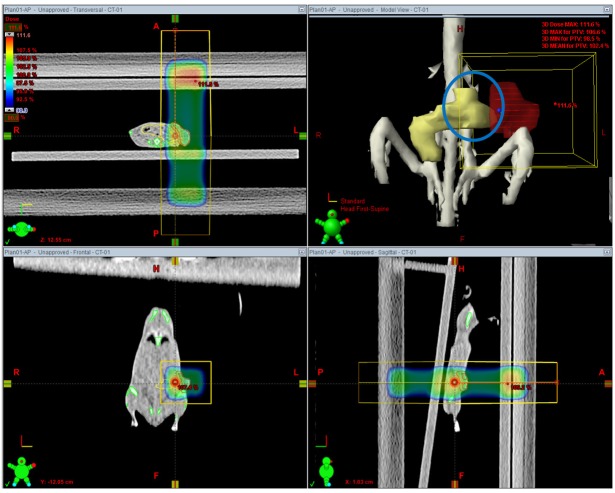
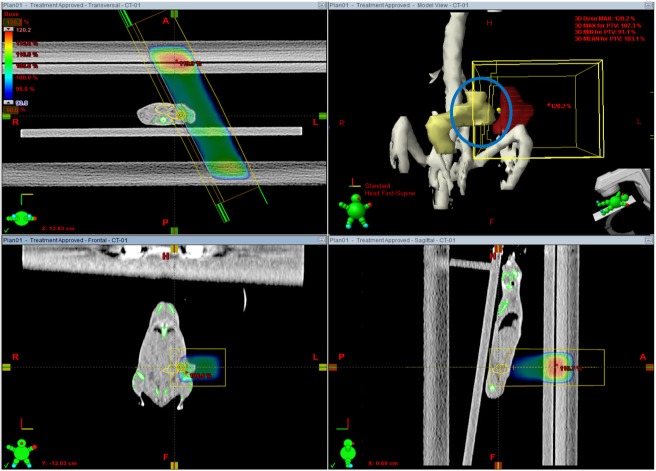
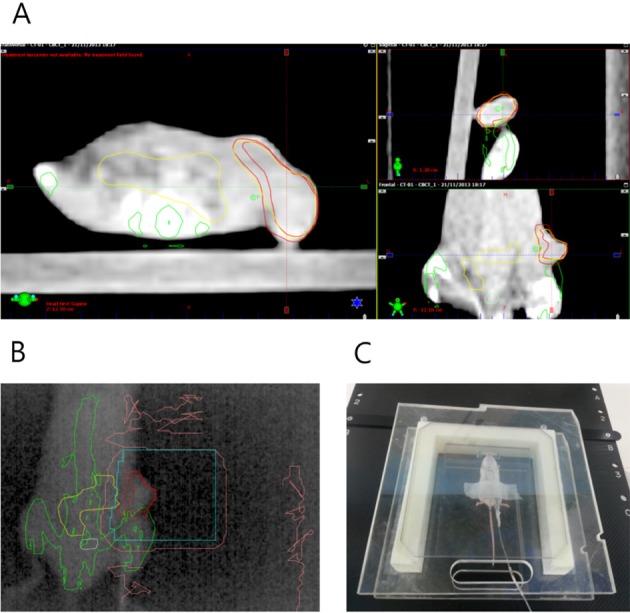
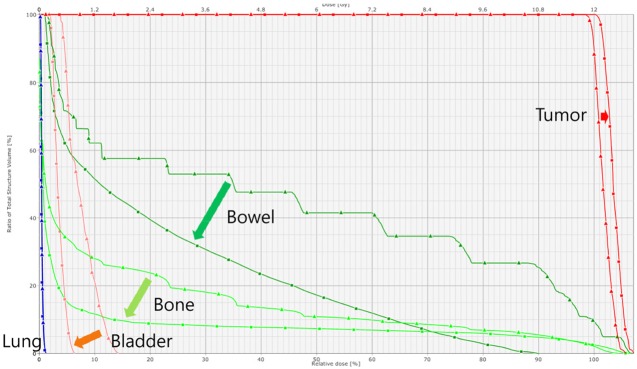
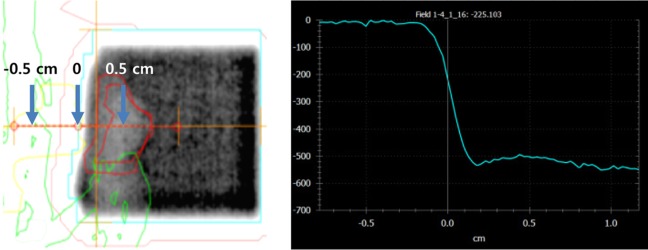
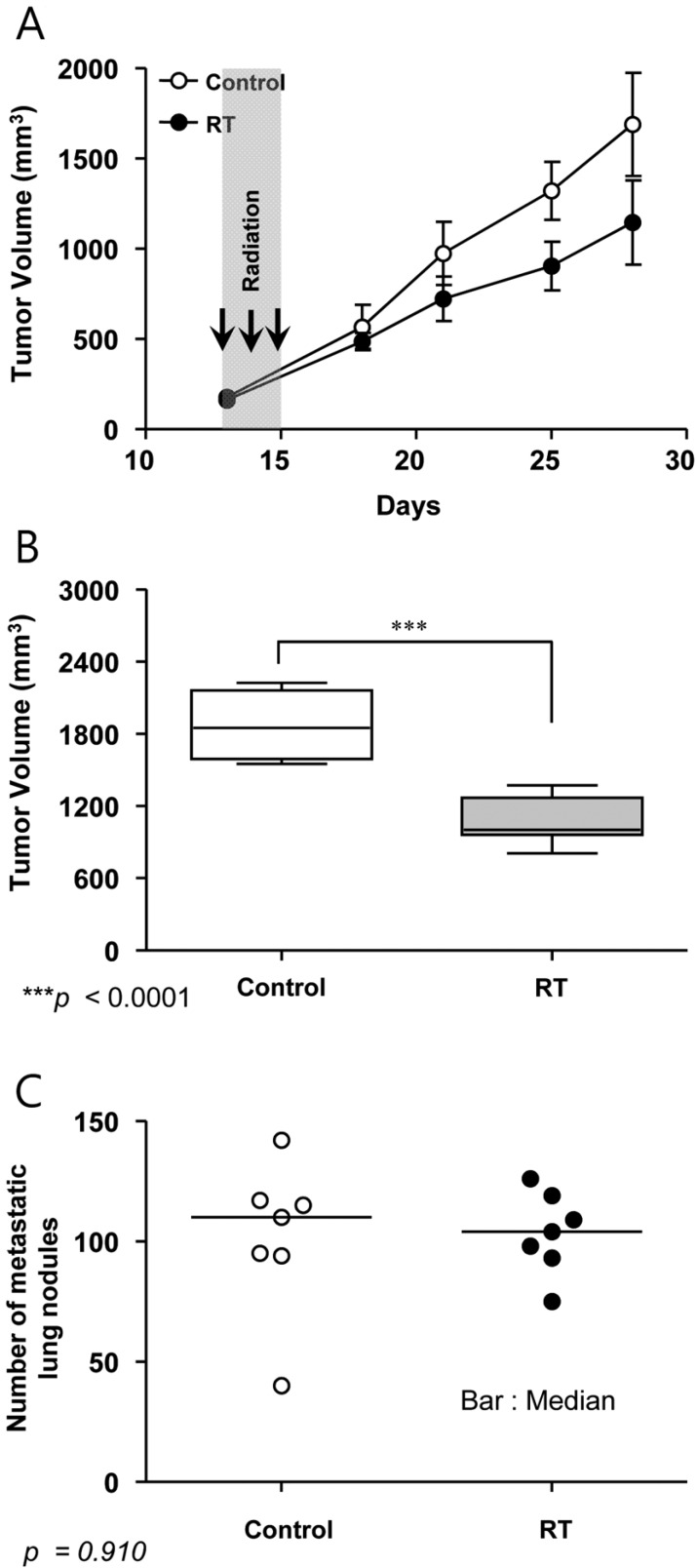
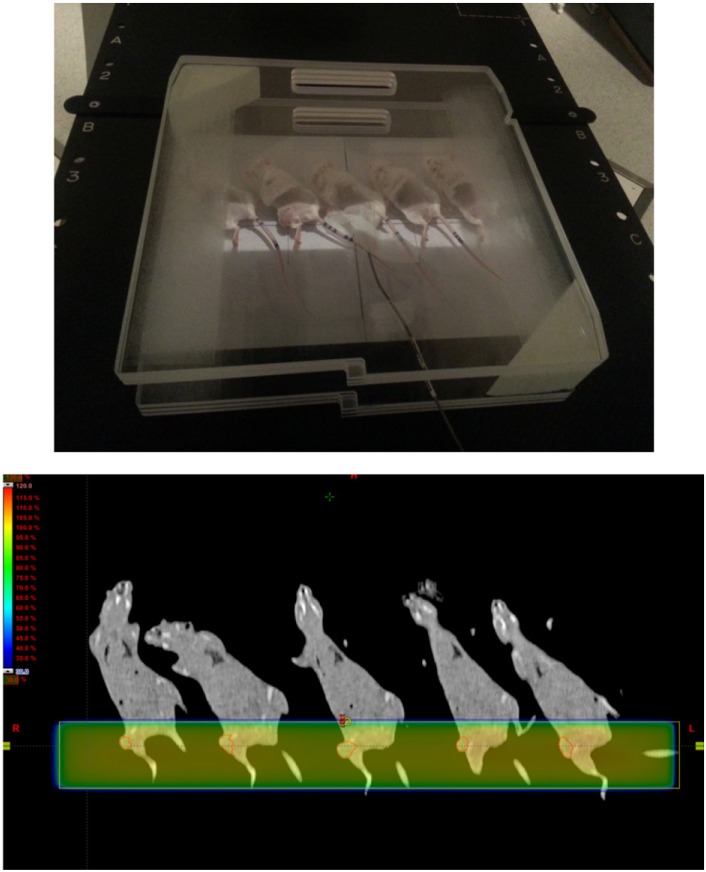
- This study was conducted to assess the feasibility of image guided radiotherapy (IGRT) for orthotopic 4T1 mouse mammary tumor using linear accelerator (LINAC). Eighteen Balb/C mice were inoculated with 4T1 cells on left mammary fat pad and nine of them were irradiated using LINAC. Tumors, planning target volumes (PTV), bowels adjacent to tumors, bones and lungs were delineated on planning CT images. IGRT plans were generated to irradiate prescription dose to at least 90% of the PTV and then compared with conventional 2-dimensional plans with anterior-posterior and posterior-anterior beams with 5 mm margins (2D AP/PA plan). Homemade dose-build-up-cradle was designed to encompass mouse bed for homogeneous dose build up. To confirm the irradiated dose, tumor doses were measured using diode detector placed on the surface of tumors. Plan comparison demonstrated equivalent doses to PTV while sparing more doses to normal tissues including bowel (from 90.9% to 40.5%, median value of mean doses) and bone marrow (from 12.9% to 4.7%, median value of mean doses) than 2D AP/PA plan. Quality assurance using diode detector confirmed that IGRT could deliver 95.3-105.3% of the planned doses to PTV. Tumors grew 505.2-1185.8% (mean 873.3%) in the control group and 436.1-771.8% (mean 615.5%) in the irradiated group. These results demonstrate that LINAC-based IGRT provides a reliable approach with accurate dose delivery in the radiobiological study for orthotropic tumor model maintaining tumor microenvironment.
Keyword
MeSH Terms
Figure
Reference
-
1. Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 1994; 13(2):209–222. PMID: 7923551.
Article2. Kuo TH, Kubota T, Watanabe M, Furukawa T, Kase S, Tanino H, Saikawa Y, Ishibiki K, Kitajima M, Hoffman RM. Site-specific chemosensitivity of human small-cell lung carcinoma growing orthotopically compared to subcutaneously in SCID mice: the importance of orthotopic models to obtain relevant drug evaluation data. Anticancer Res. 1993; 13(3):627–630. PMID: 8391244.3. Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005; 11(2 Pt 1):728–734. PMID: 15701862.4. Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009; 15(2):597–606. PMID: 19147765.
Article5. Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, Barcellos-Hoff MH. TGFâ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011; 17(21):6754–6765. PMID: 22028490.
Article6. Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, Haynes NM. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012; 72(13):3163–3174. PMID: 22570253.
Article7. Wang WC, Liang SL, Chen YK, Lin LM. The therapeutic effect of fractionated radiation on DMBA-induced hamster buccal pouch squamous cell carcinomas. Oral Oncol. 2008; 44(12):1160–1166. PMID: 18486531.
Article8. Potters L, Gaspar LE, Kavanagh B, Galvin JM, Hartford AC, Hevezi JM, Kupelian PA, Mohiden N, Samuels MA, Timmerman R, Tripuraneni P, Vlachaki MT, Xing L, Rosenthal SA. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guidelines for image-guided radiation therapy (IGRT). Int J Radiat Oncol Biol Phys. 2010; 76(2):319–325. PMID: 20117284.
Article9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144(5):646–674. PMID: 21376230.
Article10. Khan F. Clinical radiation generators. The physics of radiation therapy. 4th ed. Lippincott Williams & Wilkins;2009. p. 35–53.11. Wuerfel J. Dose measurements in small fields. Med Phys Int J. 2013; 1(1):81–90.12. Saini AS, Zhu TC. Energy dependence of commercially available diode detectors for in-vivo dosimetry. Med Phys. 2007; 34(5):1704–1711. PMID: 17555252.13. Khan F. Treatment Planning I: Isodose Distributions. The physics of radiation therapy. 4th ed. Lippincott Williams & Wilkins;2009. p. 176–200.14. Balagamwala EH, Angelov L, Koyfman SA, Suh JH, Reddy CA, Djemil T, Hunter GK, Xia P, Chao ST. Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2012; 17(6):556–564. PMID: 23020208.
Article15. Stinauer MA, Kavanagh BD, Schefter TE, Gonzalez R, Flaig T, Lewis K, Robinson W, Chidel M, Glode M, Raben D. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011; 6:34. PMID: 21477295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LINAC-based High-precision Radiotherapy: Radiosurgery, Image-guided Radiotherapy, and Respiratory-gated Radiotherapy
- Helical Tomotherapy: Image-guided Intensity Modulated Radiation Therapy
- Evolution of Radiotherapy: High-precision Radiotherapy
- Establishment of an Orthotopic Mouse Non-Muscle Invasive Bladder Cancer Model Expressing the Mammalian Target of Rapamycin Signaling Pathway
- OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer