J Vet Sci.
2013 Jun;14(2):223-226. 10.4142/jvs.2013.14.2.223.
Analysis of ultrastructural glomerular basement membrane lesions and podocytes associated with proteinuria and sclerosis in Osborne-Mendel rats with progressive glomerulonephropathy
- Affiliations
-
- 1Research Institute of Biosciences, School of Veterinary Medicine, Azabu University, Kanagawa 252-5201, Japan. shirota@azabu-u.ac.jp
- 2Laboratory of Veterinary Pathology, School of Veterinary Medicine, Azabu University, Kanagawa 252-5201, Japan.
- KMID: 1705525
- DOI: http://doi.org/10.4142/jvs.2013.14.2.223
Abstract
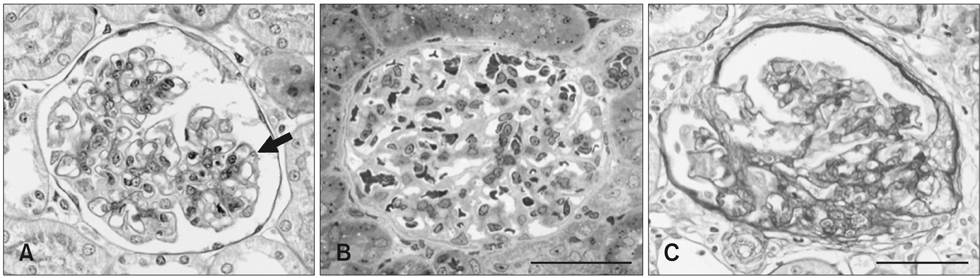
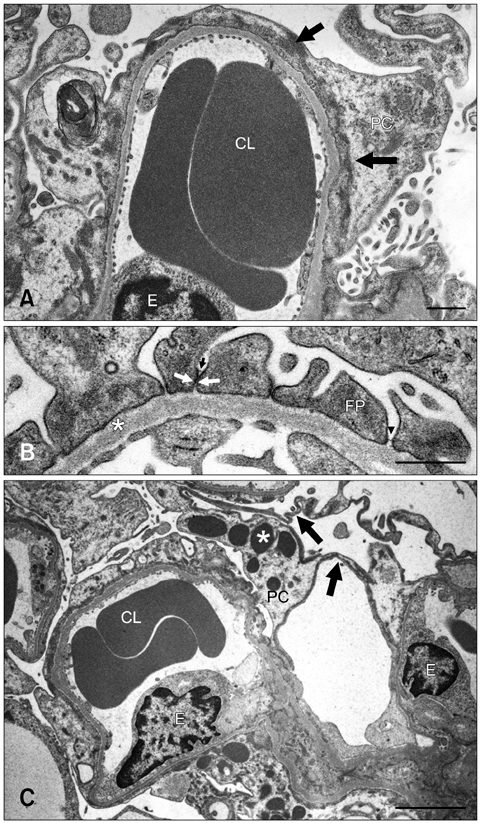
- The renal glomeruli of 12 male Osborne-Mendel (OM) rats 3 to 24 weeks old were examined by electron microscopy. Effacement of podocyte foot processes (FPs) developed at 3 weeks of age and became progressively worse over time. Loss or dislocation of the slit membrane was also found. Vacuoles and osmiophilic lysosomes appeared in the podocytes starting at 6 weeks of age. Podocyte detachment from the glomerular basement membrane (GBM) was apparent at 18 weeks of age. Laminated GBM was occasionally observed in all animals. These features might lead to the development of spontaneous proteinuria and glomerulosclerosis in OM rats.
MeSH Terms
-
Animals
Animals, Outbred Strains
Glomerular Basement Membrane/*pathology/ultrastructure
Kidney Diseases/complications/etiology/*pathology
Male
Microscopy, Electron, Transmission
Nephrosclerosis/etiology/pathology
Nephrosis/complications/pathology
Podocytes/*pathology/ultrastructure
Proteinuria/etiology/pathology
Rats
Figure
Reference
-
1. Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985; 100:1988–2000.
Article2. Gubler MC, Heidet L, Antignac C. Alport's syndrome, thin basement membrane nephropathy, nail-pattela syndrome and type III collagen glomerulopathy. In : Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Haptinstall's Pathology of the Kidney. 6th ed. New York: Lippincott Williams & Wilkins;2007. p. 487–502.3. Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008; 88:451–487.
Article4. Jansen B, Thorner P, Baumal R, Valli V, Maxie MG, Singh A. Samoyed hereditary glomerulopathy (SHG). Evolution of splitting of glomerular capillary basement membranes. Am J Pathol. 1986; 125:536–545.5. Kriz W. Progressive renal failure-inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996; 11:1738–1742.
Article6. Kriz W. The pathogenesis of 'classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003; 18:Suppl 6. vi39–vi44.
Article7. Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992; 141:805–816.8. Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G. Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005; 166:1309–1320.
Article9. Ogura A, Fujimura H, Asano T, Koura M, Naito I, Kobayashi Y. Early ultrastructural glomerular alterations in neonatal nephrotic mice (ICGN strain). Vet Pathol. 1995; 32:321–323.
Article10. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83:253–307.
Article11. Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006; 69:2131–2147.
Article12. Yasuno K, Ishihara S, Saito R, Ishikawa M, Kato T, Kobayashi R, Baba T, Kawano K, Ogihara K, Kamiie J, Shirota K. Early-onset podocyte injury and glomerular sclerosis in Osborne-Mendel rats. J Vet Med Sci. 2010; 72:1319–1327.
Article13. Yoshida S, Nagase M, Shibata S, Fujita T. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Exp Nephrol. 2008; 108:e57–e68.
Article14. Yoshikawa N, Ito H, Akamatsu R, Hazikano H, Okada S, Matsuo T. Glomerular podocytes vacuolation in focal segmental glomerulosclerosis. Arch Pathol Lab Med. 1986; 110:394–398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrastructural Changes in Glomerular Anionic Sites in Puromycin Aminonucleoside Nephropathy
- Morphological Changes in Glomerular Epithelial Cells and Basement Membranes in Puromycin Aminonucleoside Induced Nephropathy
- Transforming growth factor-beta and the glomerular filtration barrier
- Concurrent Thin Basement Membrane Disease and Minimal Change Disease: A Case Report
- Podocytopathy and Morphologic Changes in Focal Segmental Glomerulosclerosis