J Vet Sci.
2013 Jun;14(2):175-184. 10.4142/jvs.2013.14.2.175.
Evaluation of a canine small intestinal submucosal xenograft and polypropylene mesh as bioscaffolds in an abdominal full-thickness resection model of growing rats
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. hykim@konkuk.ac.kr
- 2Department of Veterinary Clinical Pathology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3School of Veterinary Medicine, University of California, Davis, CA 95616, USA.
- KMID: 1705519
- DOI: http://doi.org/10.4142/jvs.2013.14.2.175
Abstract
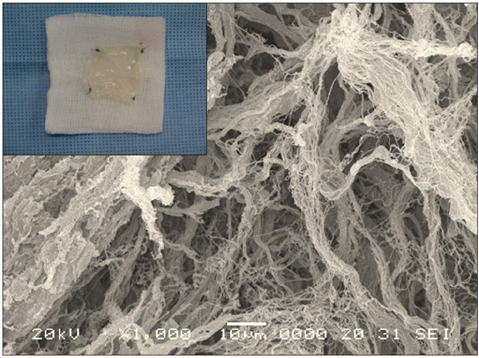
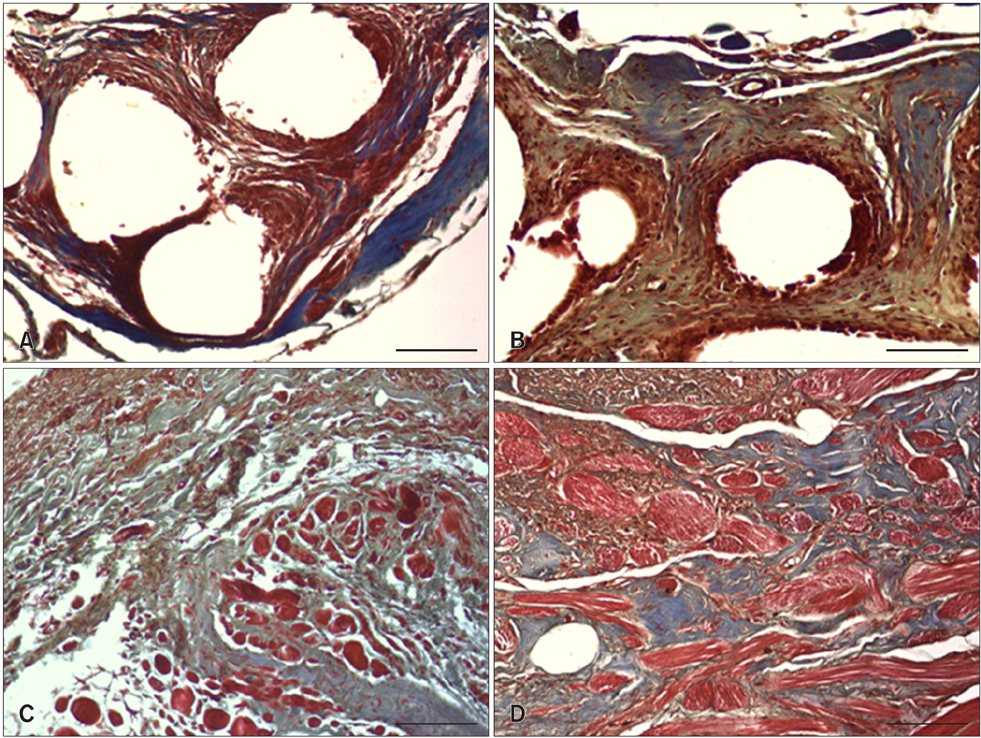
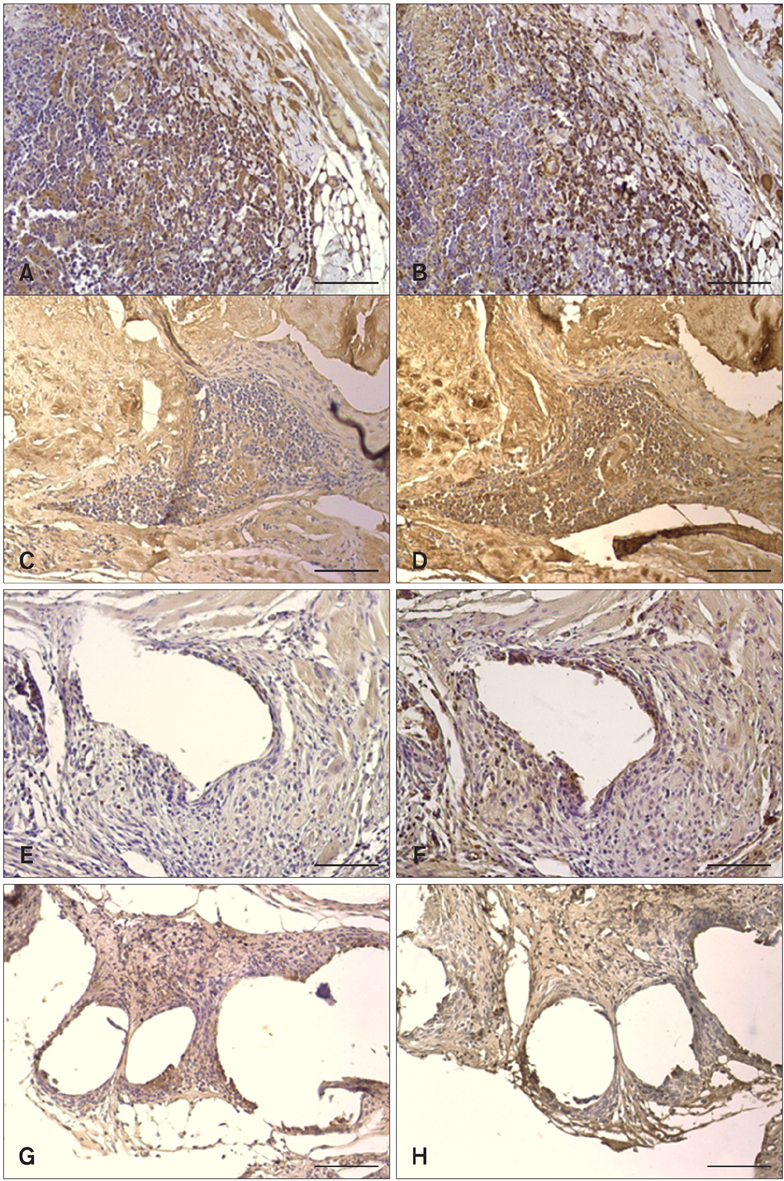
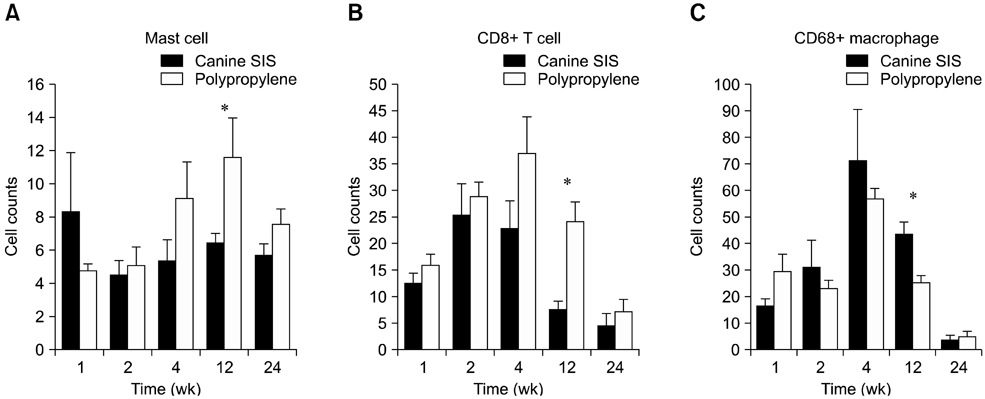
- We evaluated the biological scaffold properties of canine small intestinal submucosa (SIS) compared to a those of polypropylene mesh in growing rats with full-thickness abdominal defects. SIS is used to repair musculoskeletal tissue while promoting cell migration and supporting tissue regeneration. Polypropylene mesh is a non-resorbable synthetic material that can endure mechanical tension. Canine SIS was obtained from donor German shepherds, and its porous collagen fiber structure was identified using scanning electron microscopy (SEM). A 2.50-cm2 section of canine SIS (SIS group) or mesh (mesh group) was implanted in Sprague-Dawley rats. At 1, 2, 4, 12, and 24 weeks after surgery, the implants were histopathologically examined and tensile load was tested. One month after surgery, CD68+ macrophage numbers in the SIS group were increased, but the number of CD8+ T cells in this group declined more rapidly than that in rats treated with the mesh. In the SIS group, few adhesions and well-developed autologous abdominal muscle infiltration into the SIS collagen fibers were observed. No significant differences in the tensile load test results were found between the SIS and mesh groups at 24 weeks. Canine SIS may therefore be a suitable replacement for artificial biological scaffolds in small animals.
Keyword
MeSH Terms
-
Abdominal Wall/*surgery
Animals
Biocompatible Materials/*therapeutic use
Dogs
Female
Intestinal Mucosa/cytology/transplantation
Intestine, Small/cytology/*transplantation
Polypropylenes/*therapeutic use
Rats
Rats, Sprague-Dawley
Tensile Strength
Tissue Adhesions
*Tissue Scaffolds
Transplantation, Heterologous/*methods
*Wound Healing
Biocompatible Materials
Polypropylenes
Figure
Reference
-
1. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996; 383:787–793.
Article2. Allman AJ, McPherson TB, Merrill LC, Badylak SF, Metzger DW. The Th2-restricted immune response to xenogeneic small intestinal submucosa does not influence systemic protective immunity to viral and bacterial pathogens. Tissue Eng. 2002; 8:53–62.
Article3. Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997; 1:15–21.
Article4. Badylak SF. Small intestinal submucosa (SIS): a biomaterial conducive to smart tissue remodeling. In : Bell E, editor. Tissue engineering: current perspectives. 1st ed. Boston: Birkhäuser;1993. p. 179–189.5. Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989; 47:74–80.
Article6. Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000; 35:1097–1103.
Article7. Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995; 29:977–985.
Article8. Bystrom J, Evans I, Newson J, Stables M, Toor I, Van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008; 112:4117–4127.
Article9. Clarke KM, Lantz GC, Salisbury SK, Badylak SF, Hiles MC, Voytik SL. Intestine submucosa and polypropylene mesh for abdominal wall repair in dogs. J Surg Res. 1996; 60:107–114.
Article10. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005; 6:135–142.
Article11. Gastel JA, Muirhead WR, Lifrak JT, Fadale PD, Hulstyn MJ, Labrador DP. Meniscal tissue regeneration using a collagenous biomaterial derived from porcine small intestine submucosa. Arthroscopy. 2001; 17:151–159.
Article12. Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996; 2:209–217.
Article13. Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995; 46:396–400.
Article14. Lantz GC, Badylak SF, Hiles MC, Coffey AC, Geddes LA, Kokini K, Sandusky GE, Morff RJ. Small intestinal submucosa as a vascular graft: a review. J Invest Surg. 1993; 6:297–310.
Article15. Liang R, Woo SLY, Takakura Y, Moon DK, Jia F, Abramowitch SD. Long-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: A functional tissue engineering study. J Orthop Res. 2006; 24:811–819.
Article16. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442:997–1002.
Article17. Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg. 2000; 232:586–596.
Article18. McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-β1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003; 67:637–640.
Article19. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009; 15:914–920.
Article20. Pu LL. Small intestinal submucosa (Surgisis) as a bioactive prosthetic material for repair of abdominal wall fascial defect. Plast Reconstr Surg. 2005; 115:2127–2131.
Article21. Sandoval JA, Lou D, Engum SA, Fisher LM, Bouchard CM, Davis MM, Grosfeld JL. The whole truth: comparative analysis of diaphragmatic hernia repair using 4-ply vs 8-ply small intestinal submucosa in a growing animal model. J Pediatr Surg. 2006; 41:518–523.
Article22. Smith MJ, Paran TS, Quinn F, Corbally MT. The SIS extracellular matrix scaffold-preliminary results of use in congenital diaphragmatic hernia (CDH) repair. Pediatr Surg Int. 2004; 20:859–862.
Article23. Smith RS. The use of prosthetic materials in the repair of hernias. Surg Clin North Am. 1971; 51:1387–1399.
Article24. Suckow M, Voytik-Harbin SL, Terril LA, Badylak SF. Enhanced bone regeneration using porcine small intestinal submucosa. J Invest Surg. 1999; 12:277–287.
Article25. Verreck FAW, De Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, De Waal-Malefyt R, Ottenhoff THM. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) bacteria. Proc Natl Acad Sci U S A. 2004; 101:4560–4565.
Article26. Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg. 1981; 194:219–223.27. Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997; 67:478–491.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reconstruction of a high-energy penetrating injury from the abdomen to the sacral area using a latissimus dorsi free flap with monofilament polypropylene mesh and pedicled flap rotation: a case report
- An Ileocutaneous Fistula That Developed 12 Years after Repair of an Abdominal Wall Defect Using Intraperitoneal Placement of High-density Polypropylene Mesh (Marlex(R))
- Comparison of Polypropylene Mesh and Expanded Polytetrafluoroethylene Patch for Repair of Abdominal Wall Defects in Rat
- Totally Extraperitoneal Laparoscopic Repair of Obturator Hernia withPartial Intestinal Obstruction
- Reconstruction of the diaphragm with autologous fascia lata during cytoreduction in patients with advanced ovarian cancer