J Vet Sci.
2013 Jun;14(2):151-159. 10.4142/jvs.2013.14.2.151.
Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells
- Affiliations
-
- 1Adult Stem Cell Research Center, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr
- 2Laboratory of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 3BK 21 Program for Veterinary Sciences, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 4Laboratory of Internal Medicine, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. jschae@snu.ac.kr
- KMID: 1705516
- DOI: http://doi.org/10.4142/jvs.2013.14.2.151
Abstract
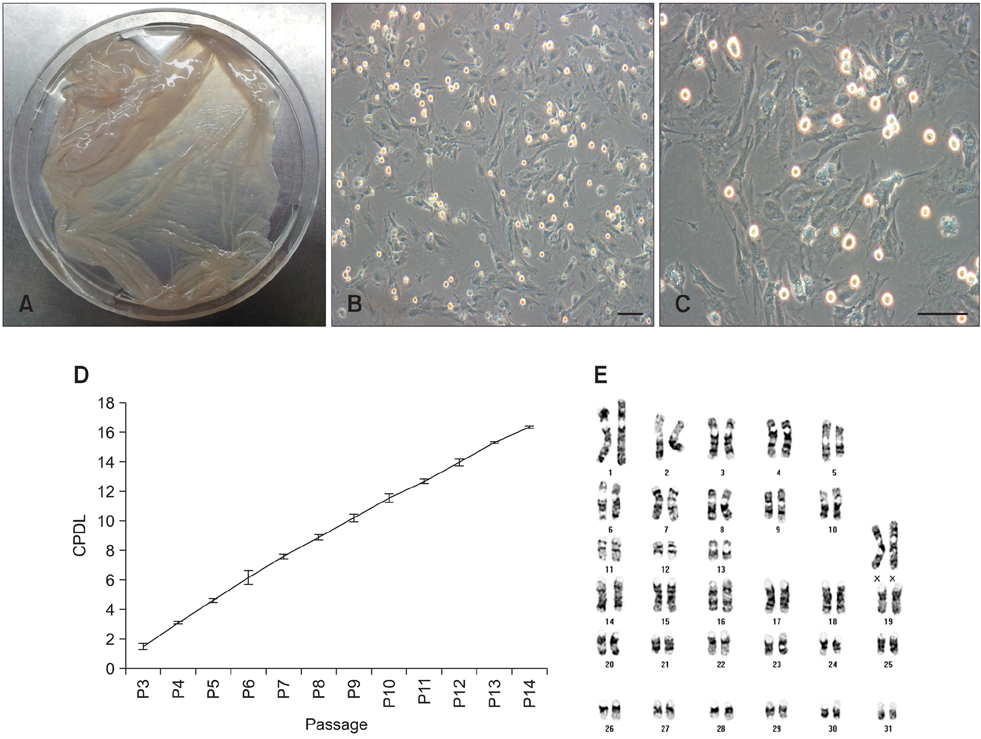
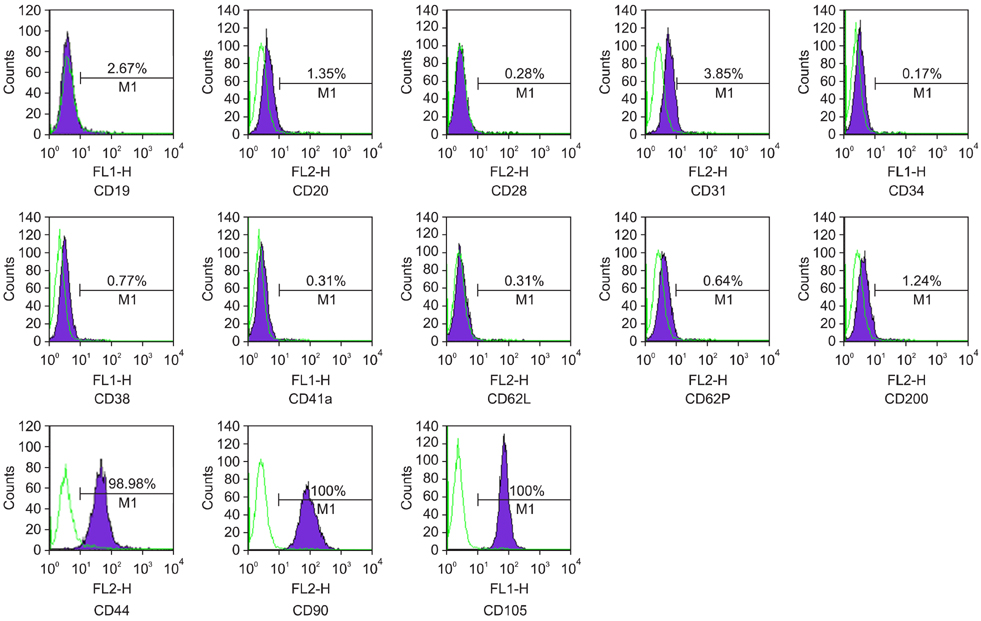
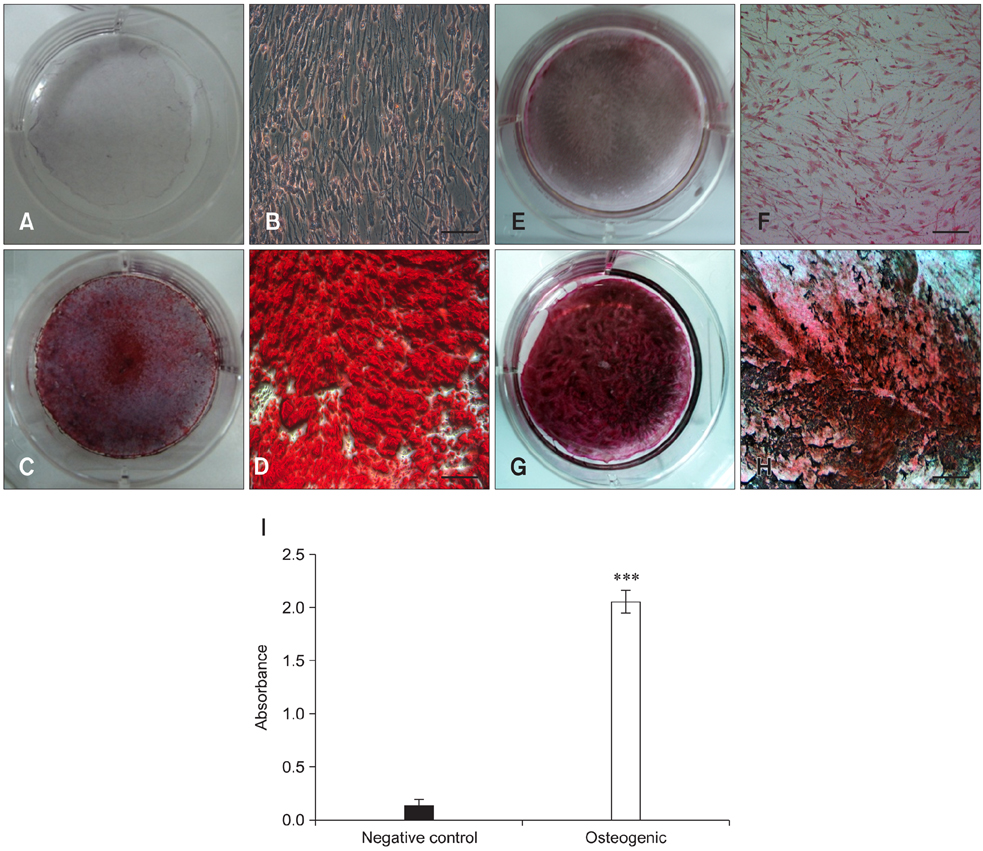
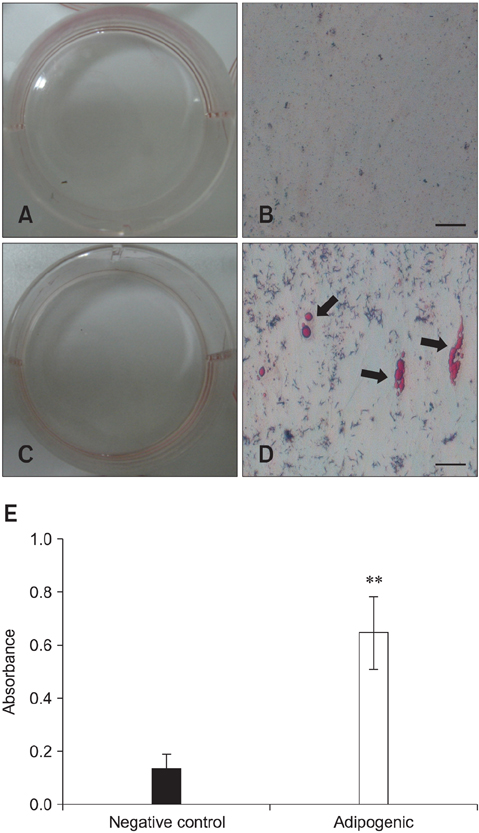
- Recent studies have shown that mesenchymal stem cells (MSCs) are able to differentiate into multi-lineage cells such as adipocytes, chondroblasts, and osteoblasts. Amniotic membrane from whole placenta is a good source of stem cells in humans. This membrane can potentially be used for wound healing and corneal surface reconstruction. Moreover, it can be easily obtained after delivery and is usually discarded as classified waste. In the present study, we successfully isolated and characterized equine amniotic membrane-derived mesenchymal stem cells (eAM-MSCs) that were cultured and maintained in low glucose Dulbecco's modified Eagle's medium. The proliferation of eAM-MSCs was measured based on the cumulative population doubling level (CPDL). Immunophenotyping of eAM-MSCs by flow cytometry showed that the major population was of mesenchymal origin. To confirm differentiation potential, a multi-lineage differentiation assay was conducted. We found that under appropriate conditions, eAM-MSCs are capable of multi-lineage differentiation. Our results indicated that eAM-MSCs may be a good source of stem cells, making them potentially useful for veterinary regenerative medicine and cell-based therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Arnhold SJ, Goletz I, Klein H, Stumpf G, Beluche LA, Rohde C, Addicks K, Litzke LF. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007; 68:1095–1105.
Article2. De Bruyn C, Najar M, Raicevic G, Meuleman N, Pieters K, Stamatopoulos B, Delforge A, Bron D, Lagneaux L. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton's jelly without enzymatic treatment. Stem Cells Dev. 2011; 20:547–557.
Article3. de Mattos Carvalho A, Alves ALG, Golim MA, Moroz A, Hussni CA, de Oliveira PGG, Deffune E. Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet Immunol Immunopathol. 2009; 132:303–306.
Article4. De Schauwer C, Meyer E, Van de Walle GR, Van Soom A. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 2011; 75:1431–1443.
Article5. Díaz-Prado S, Muiños-López E, Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ, Blanco FJ. Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng Part C Methods. 2011; 17:49–59.
Article6. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. The International Society for Cellular Therapy position statement. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006; 8:315–317.
Article7. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009; 27:1675–1680.
Article8. Kamishina H, Deng J, Oji T, Cheeseman JA, Clemmons RM. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am J Vet Res. 2006; 67:1921–1928.
Article9. Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008; 29:907–916.
Article10. Klein JD, Fauza DO. Amniotic and placental mesenchymal stem cell isolation and culture. Methods Mol Biol. 2011; 698:75–88.
Article11. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26.
Article12. La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasa L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009; 131:267–282.
Article13. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004; 103:1669–1675.
Article14. Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009; 433:1–7.
Article15. Marongiu F, Gramignoli R, Sun Q, Tahan V, Miki T, Dorko K, Ellis E, Strom SC. Isolation of amniotic mesenchymal stem cells. Curr Protoc Stem Cell Biol. 2010; 12:Suppl. 1E 5.1–1E 5.11.
Article16. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006; 30:681–687.
Article17. Mihu CM, Rus Ciucă D, Soritău O, Suşman S, Mihu D. Isolation and characterization of mesenchymal stem cells from the amniotic membrane. Rom J Morphol Embryol. 2009; 50:73–77.18. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010; 19:1449–1470.
Article19. Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008; 69:928–937.
Article20. Park SB, Seo MS, Kang JG, Chae JS, Kang KS. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy. 2011; 13:341–349.
Article21. Park SB, Seo MS, Kim HS, Kang KS. Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS One. 2012; 7:e44693.
Article22. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008; 26:300–311.
Article23. Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008; 14:181–187.
Article24. Schnabel LV, Lynch ME, van der Meulen MCH, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009; 27:1392–1398.
Article25. Takaoka M, Nakamura T, Sugai H, Bentley AJ, Nakajima N, Fullwood NJ, Yokoi N, Hyon SH, Kinoshita S. Sutureless amniotic membrane transplantation for ocular surface reconstruction with a chemically defined bioadhesive. Biomaterials. 2008; 29:2923–2931.
Article26. Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010; 19:279–289.
Article27. Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007; 25:913–925.
Article28. Yu SJ, Soncini M, Kaneko Y, Hess DC, Parolini O, Borlongan CV. Amnion: a potent graft source for cell therapy in stroke. Cell Transplant. 2009; 18:111–118.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Mini Overview of Isolation, Characterization and Application of Amniotic Fluid Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Adipose Tissue Derived Mesenchymal Stem Cells
- ERRATUM: Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells