J Vet Sci.
2013 Jun;14(2):143-150. 10.4142/jvs.2013.14.2.143.
Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticle-based extraction and an enzyme-linked immunosorbent assay
- Affiliations
-
- 1Animal and Plant Quarantine Agency, Anyang 430-757, Korea. kanghg67@korea.kr
- 2Nanobrick Co., Ltd, Suwon 443-270, Korea.
- 3Analytical Toxicology Lab, College of Veterinary Medicine, Cornell University, NY 14850, USA.
- 4Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Seoul 157-742, Korea. mchotox@snu.ac.kr
- KMID: 1705515
- DOI: http://doi.org/10.4142/jvs.2013.14.2.143
Abstract
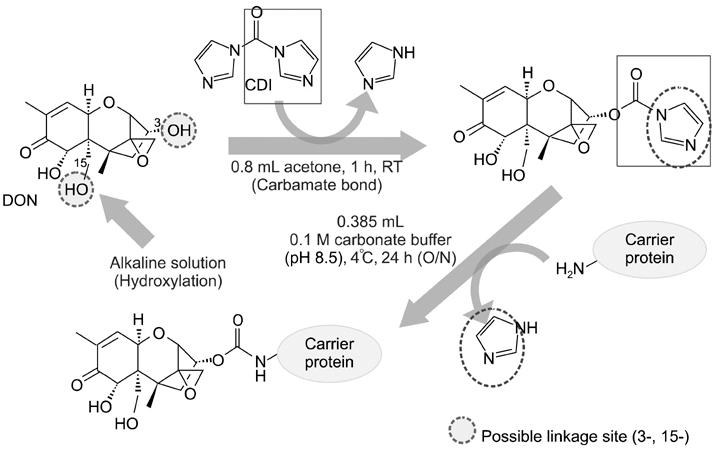
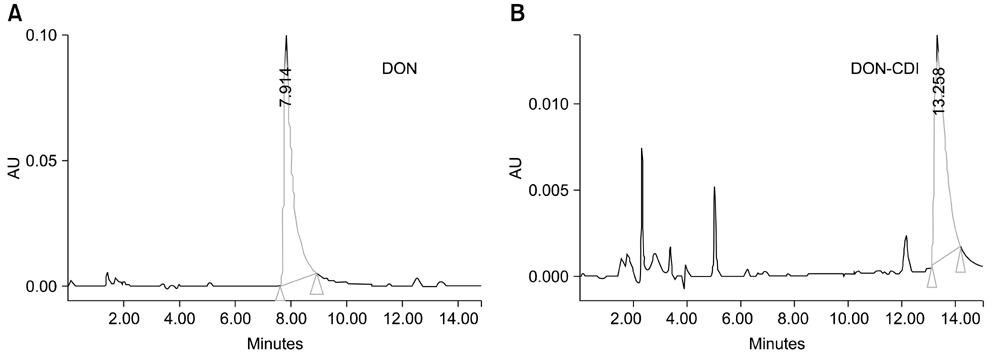
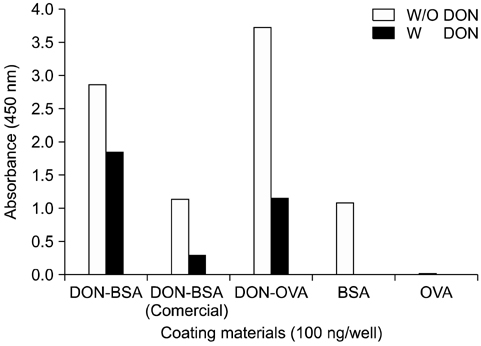
- Monoclonal antibody (mAb, NVRQS-DON) against deoxynivalenol (DON) was prepared. DON-Ag coated enzyme linked immunosorbent assay (ELISA) and DON-Ab coated ELISA were prepared by coating the DON-BSA and DON mAb. Quantitative DON calculation ranged from 50 to 4,000 ng/mL for DON-Ab coated ELISA and from 25 to 500 ng/mL for DON-Ag coated ELISA. 50% of inhibitory concentration values of DON, HT-2, 15-acetyl-DON, and nivalenol were 23.44, 22,545, 5,518 and 5,976 ng/mL based on the DON-Ab coated ELISA. Cross-reactivity levels of the mAb to HT-2, 15-acetyl-DON, and nivalenol were 0.1, 0.42, and 0.40%. The intra- and interassay precision coefficient variation (CV) were both <10%. In the mAb-coated ELISA, mean DON recovery rates in animal feed (0 to 1,000 microg/kg) ranged from 68.34 to 95.49% (CV; 4.10 to 13.38%). DON in a buffer solution (250, 500 and 1,000 ng/mL) was isolated using 300 microg of NVRQS-DON and 3 mg of magnetic nanoparticles (MNPs). The mean recovery rates of DON using this mAb-MNP system were 75.2, 96.9, and 88.1% in a buffer solution spiked with DON (250, 500, and 1,000 ng/mL). Conclusively we developed competitive ELISAs for detecting DON in animal feed and created a new tool for DON extraction using mAb-coupled MNPs.
MeSH Terms
-
Animal Feed/analysis
Animals
Antibodies, Fungal/analysis
Antibodies, Monoclonal/analysis
Chemistry Techniques, Analytical/*methods
Enzyme-Linked Immunosorbent Assay/*methods/veterinary
Female
Food Contamination/*analysis
Fusarium/immunology
Imidazoles/chemistry
Magnetics/methods
Mice
Mice, Inbred BALB C
Mycotoxins/*analysis/chemistry
Nanoparticles/chemistry
Ovalbumin/chemistry
Trichothecenes/*analysis/chemistry
Antibodies, Fungal
Antibodies, Monoclonal
Imidazoles
Mycotoxins
Ovalbumin
Trichothecenes
Figure
Cited by 1 articles
-
Magnetic nanoparticle based purification and enzyme-linked immunosorbent assay using monoclonal antibody against enrofloxacin
Nam-Gun Kim, Myeong-Ae Kim, Young-Il Park, Tae-Sung Jung, Seong-Wan Son, ByungJae So, Hwan-Goo Kang
J Vet Sci. 2015;16(4):431-437. doi: 10.4142/jvs.2015.16.4.431.
Reference
-
1. Böhm C, Cichna-Markl M, Brenn-Struckhofova Z, Razzazi-Fazeli E. Development of a selective sample clean-up method based on immuno-ultrafiltration for the determination of deoxynivalenol in maize. J Chromatogr A. 2008; 1202:111–117.
Article2. Bruno JG. In vitro selection of DNA to chloroaromatics using magnetic microbead-based affinity separation and fluorescence detection. Biochem Biophys Res Commun. 1997; 234:117–120.
Article3. Burkin AA, Kononenko GP, Soboleva NA. Group-specific antibodies against zearalenone and its metabolites and synthetic analogs. Prikl Biokhim Mikrobiol. 2002; 38:194–202.4. Choi GH, Lee DH, Min WK, Cho YJ, Kweon DH, Son DH, Park K, Seo JH. Cloning, expression, and characterization of single-chain variable fragment antibody against mycotoxin deoxynivalenol in recombinant Escherichia coli. Protein Expr Purif. 2004; 35:84–92.
Article5. Debotton N, Zer H, Parnes M, Harush-Frenkel O, Kadouche J, Benita S. A quantitative evaluation of the molecular binding affinity between a monoclonal antibody conjugated to a nanoparticle and an antigen by surface Plasmon resonance. Eur J Pharm Biopharm. 2010; 74:148–156.
Article6. Doyle PJ, Arbabi-Ghahroudi M, Gaudette N, Furzer G, Savard ME, Gleddie S, McLean MD, Mackenzie CR, Hall JC. Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Mol Immunol. 2008; 45:3703–3713.
Article7. Forsell JH, Witt MF, Tai JH, Jensen R, Pestka JJ. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986; 24:213–219.
Article8. Gao P, Yao S, Zhang B, Li E, Chang J. On-chip magnetic separation of microcantilever immunosensor based on the CdSe QDs-tagged magnetic microbead. Sheng Wu Gong Cheng Xue Bao. 2008; 24:315–322.9. Gong JL, Liang Y, Huang Y, Chen JW, Jiang JH, Shen GL, Yu RQ. Ag/SiO2 core-shell nanoparticle-based surface-enhanced Raman probes for immunoassay of cancer marker using silica-coated magnetic nanoparticles as separation tools. Biosens Bioelectron. 2007; 22:1501–1507.
Article10. Ji F, Chen Z, Xu J, Lu Q, Shi J. Development of the monoclonal antibody to deoxynivalenol. Wei Sheng Wu Xue Bao. 2008; 48:929–934.11. Khaydarov RA, Khaydarov RR, Gapurova O. Water purification from metal ions using carbon nanoparticle-conjugated polymer nanocomposites. Water Res. 2010; 44:1927–1933.
Article12. Klinglmayr C, Nöbauer K, Razzazi-Fazeli E, Cichna-Markl M. Determination of deoxynivalenol in organic and conventional food and feed by sol-gel immunoaffinity chromatography and HPLC-UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:187–193.
Article13. Kolosova AY, Sibanda L, Dumoulin F, Lewis J, Duveiller E, Van Peteghem C, De Saeger S. Lateral-flow colloidal gold-based immunoassay for the rapid detection of deoxynivalenol with two indicator ranges. Anal Chim Acta. 2008; 616:235–244.
Article14. Lin JJ, Chen JS, Huang SJ, Ko JH, Wang YM, Chen TL, Wang LF. Folic acid-pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009; 30:5114–5124.
Article15. Liu BH, Tsao ZJ, Wang JJ, Yu FY. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal Chem. 2008; 80:7029–7035.
Article16. Maragos C, Busman M, Sugita-Konishi Y. Production and characterization of a monoclonal antibody that cross-reacts with the mycotoxins nivalenol and 4-deoxynivalenol. Food Addit Contam. 2006; 23:816–825.
Article17. Maragos CM, MaCormick SP. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric Immunol. 2000; 12:181–192.
Article18. Plattner RD. HPLC/MS analysis of Fusarium mycotoxins, fumonisins and deoxynivalenol. Nat Toxins. 1999; 7:365–370.19. Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, Oram DH, Jacobs IJ, Shepherd JH, Reznek RH. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005; 23:2813–2821.
Article20. Ruiz-Hernández E, Baeza A, Vallet-Regí M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano. 2011; 5:1259–1266.
Article21. Shim WB, Choi JG, Kim JY, Yang ZY, Lee KH, Kim MG, Ha SD, Kim KS, Kim KY, Kim CH, Eremin SA, Chung DH. Enhanced rapidity for qualitative detection of Listeria monocytogenes using an enzyme-linked immunosorbent assay and immunochromatography strip test combined with immunomagnetic bead separation. J Food Prot. 2008; 71:781–789.
Article22. Shiu CM, Wang JJ, Yu FY. Sensitive enzyme-linked immunosorbent assay and rapid one-step immunochromatographic strip for fumonisin B1 in grain-based food and feed samples. J Sci Food Agric. 2010; 90:1020–1026.
Article23. Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L, Kizek R. Deoxynivalenol and its toxicity. Interdiscip Toxicol. 2010; 3:94–99.
Article24. Sparbier K, Asperger A, Resemann A, Kessler I, Koch S, Wenzel T, Stein G, Vorwerg L, Suckau D, Kostrzewa M. Analysis of glycoproteins in human serum by means of glycospecific magnetic bead separation and LC-MALDI-TOF/TOF analysis with automated glycopeptide detection. J Biomol Tech. 2007; 18:252–258.25. Tse LC, Dumaswala UJ, Greenwalt TJ. Separation of mixed red cell populations by using microbead columns. J Lab Clin Med. 1996; 127:489–493.
Article26. Varshney M, Yang L, Su XL, Li Y. Magnetic nanoparticle-antibody conjugates for the separation of Escherichia coli O157 : H7 in ground beef. J Food Prot. 2005; 68:1804–1811.
Article27. Wynick D, Bloom SR. Magnetic bead separation of anterior pituitary cells. Neuroendocrinology. 1990; 52:560–565.
Article28. Yuan Q, Pestka JJ, Hespenheide BM, Kuhn LA, Linz JE, Hart LP. Identification of mimotope peptides which bind to the mycotoxin deoxynivalenol-specific monoclonal antibody. Appl Environ Microbiol. 1999; 65:3279–3286.
Article29. Zhao W, Wang L, Tan W. Fluorescent nanoparticle for bacteria and DNA detection. Adv Exp Med Biol. 2007; 620:129–135.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic nanoparticle based purification and enzyme-linked immunosorbent assay using monoclonal antibody against enrofloxacin
- Detection of anti-borrelia burgdorferi antibody by enzyme-linked immunosorbent assay in Korea
- Application of aptamers for assessment of vaccine efficacy
- Comparison of enzyme-linked immunosorbent assay, indirect immunofluorescent antibody test and indirect immunoperoxidase antibody test in setecting antibodies to rickettsia tsutsugamushi
- Study on the ELISA method using monoclonal antibody for the detection of Hantaan virus