J Vet Sci.
2013 Jun;14(2):125-134. 10.4142/jvs.2013.14.2.125.
In vitro effects of meloxicam on the number, Foxp3 expression, production of selected cytokines, and apoptosis of bovine CD25+CD4+ and CD25-CD4+ cells
- Affiliations
-
- 1Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Warmia and Mazury, Olsztyn 10-718, Poland. tomasz.maslanka@uwm.edu.pl
- KMID: 1705513
- DOI: http://doi.org/10.4142/jvs.2013.14.2.125
Abstract
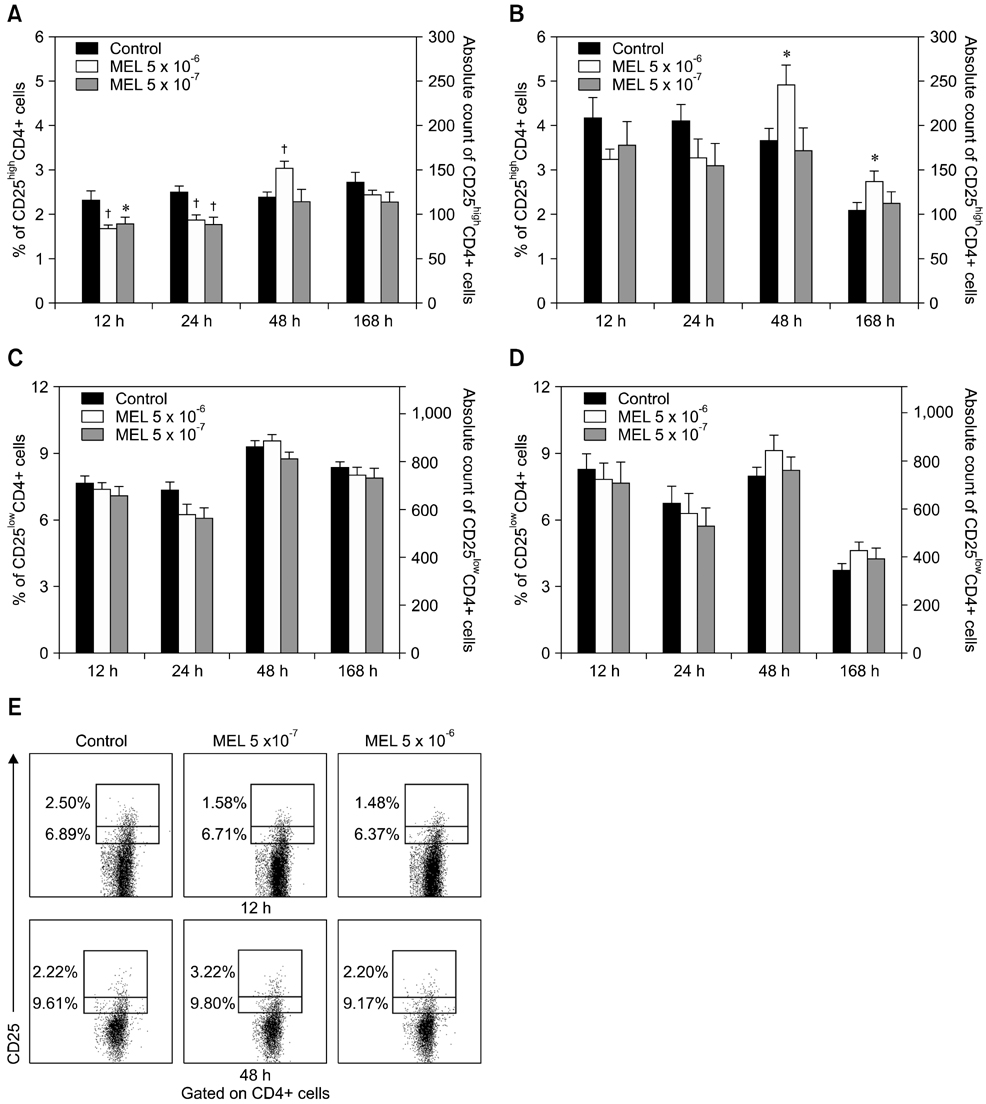
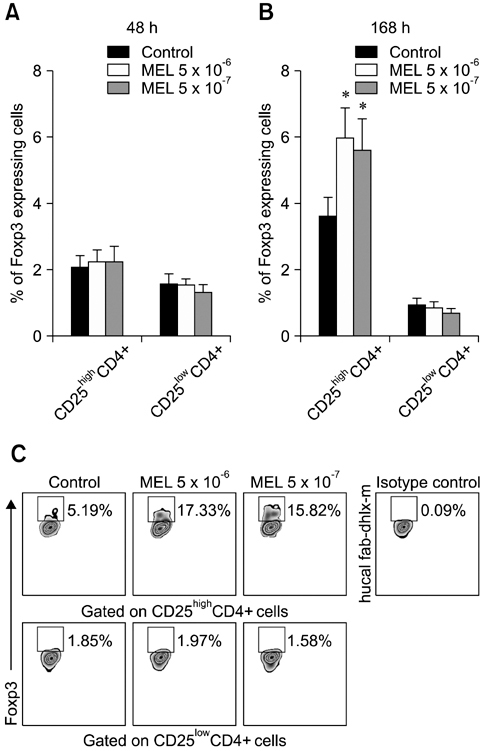
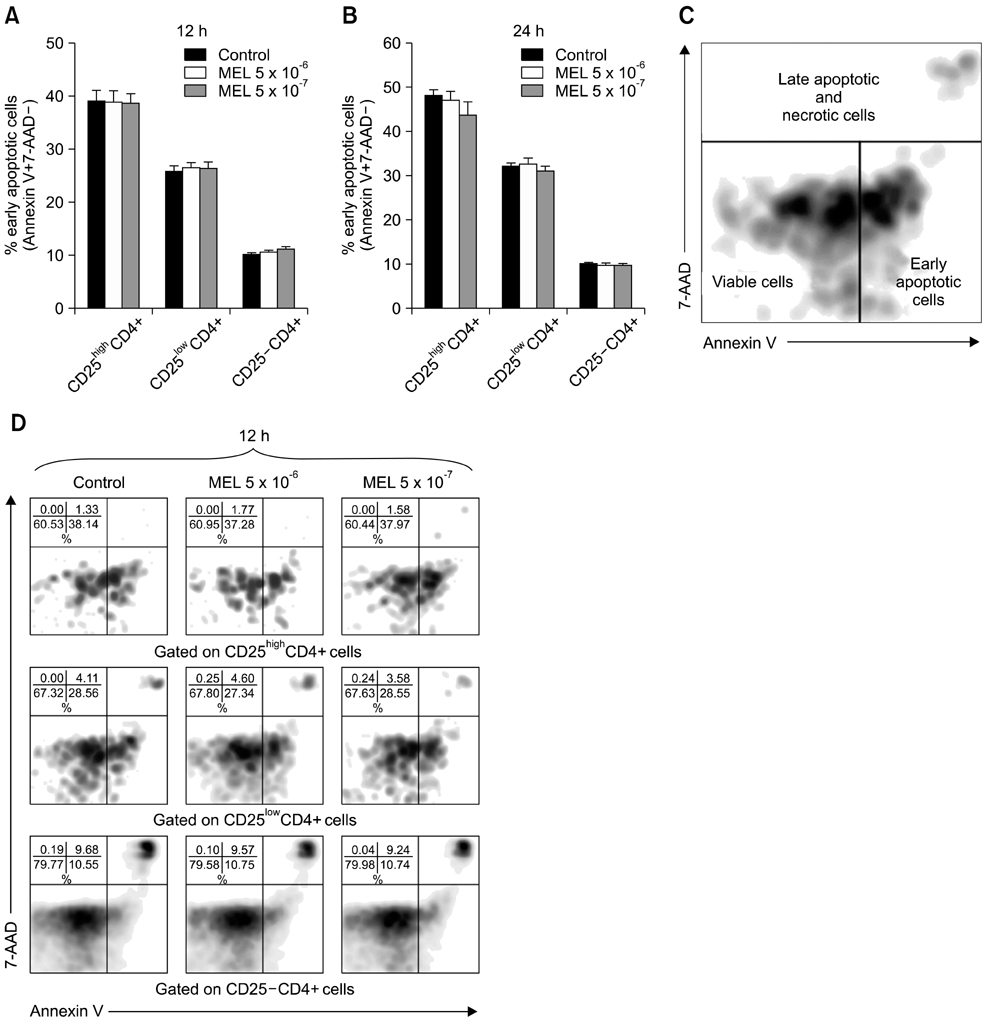
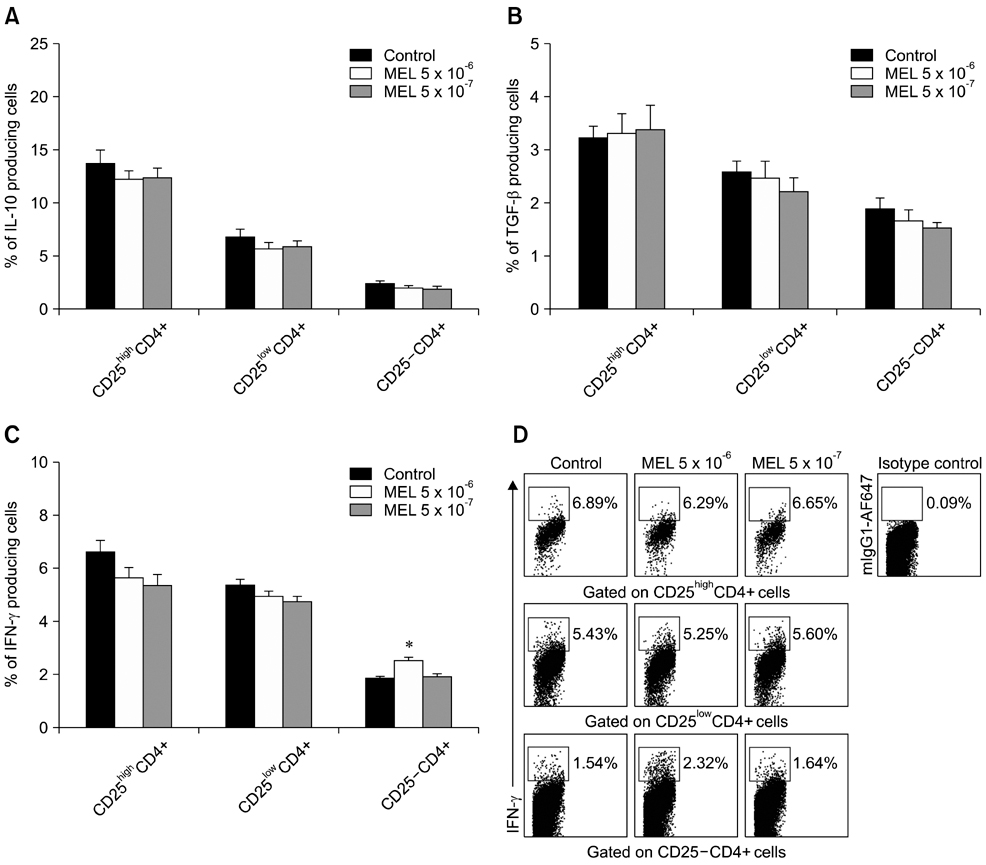
- The purpose of this study was to evaluate the effect of meloxicam (MEL) on selected immune parameters of bovine CD25highCD4+, CD25lowCD4+, and CD25-CD4+ cells. Peripheral blood mononuclear cells (PBMCs) collected from 12-month-old heifers were treated with MEL at a concentration corresponding to the serum level of this medication following administration at the recommended dose (MEL 5 x 10(-6) M) and at a concentration 10 times lower (MEL 5 x 10(-7) M). After 12 and 24 h of incubation with the drug, the percentage of CD25highCD4+ cells decreased; however, this disturbance was quickly reversed. Furthermore, the absolute number of CD25highCD4+ cells in the PBMC populations treated with MEL 5 x 10(-6) M for 48 and 168 h was increased. Prolonged (168 h) exposure to the drug increased the percentage of Foxp3+ cells in the CD25highCD4+ cell subpopulation. The higher dose of MEL was found to significantly increase the percentage of IFN-gamma+ cells among the CD25-CD4+ cells. These results indicated that MEL does not exert an immunosuppressive effect by depleting CD4+ cells and suppression of IFN-gamma+ production by these cells. Furthermore, IL-10 and TGF-beta production was not changed following exposure to MEL.
Keyword
MeSH Terms
-
Animals
Anti-Inflammatory Agents, Non-Steroidal/administration & dosage/*pharmacology
Apoptosis/*drug effects
CD4-Positive T-Lymphocytes/*drug effects/metabolism
Cattle
Cytokines/metabolism
Dose-Response Relationship, Drug
Female
Forkhead Transcription Factors/*genetics/metabolism
Gene Expression Regulation/*drug effects
Immune Tolerance/drug effects
Interleukin-2 Receptor alpha Subunit/*metabolism
Leukocytes, Mononuclear/drug effects/metabolism
Thiazines/administration & dosage/*pharmacology
Thiazoles/administration & dosage/*pharmacology
Anti-Inflammatory Agents, Non-Steroidal
Cytokines
Forkhead Transcription Factors
Interleukin-2 Receptor alpha Subunit
Thiazines
Thiazoles
Figure
Reference
-
1. Atarashi K, Mori T, Yoshiki R, Kabashima K, Kuma H, Tokura Y. Skin application of ketoprofen systemically suppresses contact hypersensitivity by inducing CD4+ CD25+ regulatory T cells. J Dermatol Sci. 2009; 53:216–221.
Article2. Bednarek D, Zdzisińska B, Kondracki M, Kandefer-Szerszeń M. Effect of steroidal and non-steroidal anti-inflammatory drugs in combination with long-acting oxytetracycline on non-specific immunity of calves suffering from enzootic bronchopneumonia. Vet Microbiol. 2003; 96:53–67.
Article3. Braitch M, Harikrishnan S, Robins RA, Nichols C, Fahey AJ, Showe L, Constantinescu CS. Glucocorticoids increase CD4+ CD25high cell percentage and Foxp3 expression in patients with multiple sclerosis. Acta Neurol Scand. 2009; 119:239–245.
Article4. Chávez E, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Castro-Sánchez L, Salazar EP, Moreno MG, Muriel P. Antifibrotic and fibrolytic properties of celecoxib in liver damage induced by carbon tetrachloride in the rat. Liver Int. 2010; 30:969–978.
Article5. Coetzee J, Kukanich B, Mosher R, Allen P. Pharmacokinetics of intravenous and oral meloxicam in ruminant calves. Vet Ther. 2009; 10:E1–E8.6. Friton GM, Cajal C, Ramirez-Romero R. Long-term effects of meloxicam in the treatment of respiratory disease in fattening cattle. Vet Rec. 2005; 156:809–811.
Article7. Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002; 168:2255–2263.
Article8. Hoek A, Rutten VPMG, Kool J, Arkesteijn GJA, Bouwstra RJ, Van Rhijn I, Koets AP. Subpopulations of bovine WC1+ γδ T cells rather than CD4+CD25highFoxp3+ T cells act as immune regulatory cells ex vivo. Vet Res. 2009; 40:6.9. Javeed A, Zhang B, Qu Y, Zhang A, Sun C, Zhang L, Liu J, Zeng C, Zhao Y. The significantly enhanced frequency of functional CD4+CD25+Foxp3+ T regulatory cells in therapeutic dose aspirin-treated mice. Transpl Immunol. 2009; 20:253–260.
Article10. Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-γ and IL-2, but not IL-4 and IL-5. J Immunol. 1995; 155:4604–4612.11. Kobayashi M, Nakamura S, Shibata K, Sahara N, Shigeno K, Shinjo K, Naito K, Ohnishi K. Etodolac inhibits EBER expression and induces Bcl-2-regulated apoptosis in Burkitt's lymphoma cells. Eur J Haematol. 2005; 75:212–220.
Article12. Liu H, Peng Y, Liu F, Li J, Chen X, Liu Y, Zhang H. A selective cyclooxygenase-2 inhibitor decreases transforming growth factor-β1 synthesis and matrix production in human peritoneal mesothelial cells. Cell Biol Int. 2007; 31:508–515.
Article13. Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, Lundholm K. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun. 2008; 8:5.14. Maeda Y, Tanaka R, Ohtsuka H, Matsuda K, Tanabe T, Oikawa M. Comparison of the immunosuppressive effects of dexamethasone, flunixin meglumine, and meloxicam on the in vitro response of calf peripheral blood mononuclear cells. J Vet Med Sci. 2011; 73:957–960.
Article15. Mahic M, Yaqub S, Johansson CC, Taskén K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006; 177:246–254.
Article16. Maślanka T, Jaroszewski JJ. Dexamethasone, but not meloxicam, suppresses proliferation of bovine CD25+CD4+ and CD25-CD4+ cells. Pol J Vet Sci. 2013; 16:219–222.17. Maślanka T, Jaroszewski JJ. In vitro effects of dexamethasone on bovine CD25+CD4+ and CD25-CD4+ cells. Res Vet Sci. 2012; 93:1367–1379.18. Maślanka T, Jaroszewski JJ, Markiewicz W, Jakubowski P. Evaluation of the influence of meloxicam and flunixin meglumine on the apoptosis of peripheral blood CD4+ and CD8+ T cells in calves. Pol J Vet Sci. 2010; 13:3–12.19. Michelin MA, Figueiredo F, Cunha FQ. Involvement of prostaglandins in the immunosuppression occurring during experimental infection by Paracoccidioides brasiliensis. Exp Parasitol. 2002; 102:170–177.
Article20. Michelin MA, Silva JS, Cunha FQ. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol. 2005; 111:71–79.
Article21. Oliveira LGR, Kuehn CC, Santos CD, Toldo MPA, do Prado JC Jr. Enhanced protection by melatonin and meloxicam combination in experimental infection by Trypanosoma cruzi. Parasite Immunol. 2010; 32:245–251.
Article22. Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009; 64:1539–1546.
Article23. Sharma S, Zhu L, Yang SC, Zhang L, Lin J, Hillinger S, Gardner B, Reckamp K, Strieter RM, Huang M, Batra RK, Dubinett SM. Cyclooxygenase 2 inhibition promotes IFN-γ-dependent enhancement of antitumor responses. J Immunol. 2005; 175:813–819.
Article24. Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004; 22:531–562.25. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003; 110:255–258.
Article26. Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DAA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009; 66:2603–2622.
Article27. Xie Y, Wu M, Song R, Ma J, Shi Y, Qin W, Jin Y. A glucocorticoid amplifies IL-2-induced selective expansion of CD4+CD25+FOXP3+ regulatory T cells in vivo and suppresses graft-versus-host disease after allogeneic lymphocyte transplantation. Acta Biochim Biophys Sin (Shanghai). 2009; 41:781–791.
Article28. Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008; 57:813–821.
Article29. Yokoyama T, Aramaki O, Takayama T, Takano S, Zhang Q, Shimazu M, Kitajima M, Ikeda Y, Shirasugi N, Niimi M. Selective cyclooxygenase 2 inhibitor induces indefinite survival of fully allogeneic cardiac grafts and generates CD4+ regulatory cells. J Thorac Cardiovasc Surg. 2005; 130:1167–1174.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- Alteration of CD4+CD25+Foxp3+ T cell level in Kawasaki disease
- IL-4 Induces CD4+CD25+ Regulatory T Cells from CD4+CD25- T Cells in Peripheral Blood