Korean J Radiol.
2013 Jun;14(3):403-411. 10.3348/kjr.2013.14.3.403.
Dual Switching Monopolar Radiofrequency Ablation Using a Separable Clustered Electrode: Comparison with Consecutive and Switching Monopolar Modes in Ex Vivo Bovine Livers
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul 110-744, Korea. jmsh@snu.ac.kr
- 2Institute of Radiation Medicine, Seoul National University College of Medicine, Seoul 110-744, Korea.
- KMID: 1705451
- DOI: http://doi.org/10.3348/kjr.2013.14.3.403
Abstract
OBJECTIVE
To compare the in-vitro efficiency of dual-switching monopolar (DSM) radiofrequency ablation (RFA) using a separable clustered electrode (Octopus(R) electrodes) with consecutive monopolar (CM) and switching monopolar (SM) RFA techniques to create an ablative zone in the explanted bovine liver.
MATERIALS AND METHODS
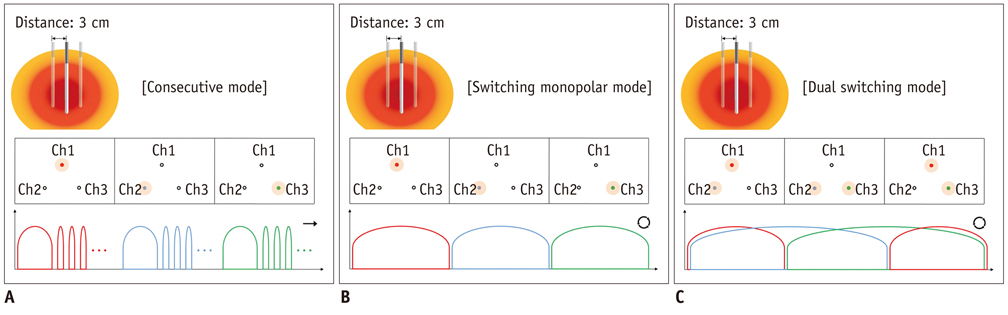
For DSM-RFA, we used a prototype, three-channel, dual generator RFA Unit and Octopus(R) electrodes with three, 17 gauge internally cooled electrodes. The RFA Unit allowed simultaneous radiofrequency (RF) energy delivery to two electrodes of the Octopus(R) electrodes as well as automatic switching among the three electrode pairs according to the impedance changes. RF energy was sequentially applied to one of the three electrodes for 24 minutes (group A; CM mode, n = 10) or alternatively applied for 12 minutes (group B; SM mode, n = 10) or concurrently applied to a pair of electrodes for 12 minutes (group C; DSM mode, n = 10) in explanted bovine livers. Changes in the impedance and current during RFA as well as the dimensions of the thermal ablative zones were compared among the three groups.
RESULTS
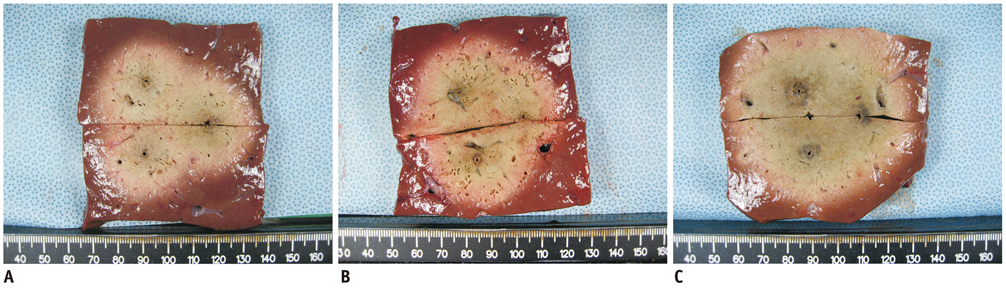
The mean, delivered RF energy amounts in groups A, B, and C were 63.15 +/- 8.6 kJ, 72.13 +/- 5.4 kJ, and 106.08 +/- 13.4 kJ, respectively (p < 0.001). The DSM mode created a significantly larger ablation volume than did the other modes, i.e., 68.1 +/- 10.2 cm3 (group A), 92.0 +/- 19.9 cm3 (group B), and 115.1 +/- 14.0 cm3 (group C) (p < 0.001). The circularity in groups A, B, and C were 0.84 +/- 0.06, 0.87 +/- 0.04 and 0.90 +/- 0.03, respectively (p = 0.03).
CONCLUSION
DSM-RFA using Octopus(R) electrodes can help create large ablative zones within a relatively short time.
MeSH Terms
Figure
Reference
-
1. Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol. 2011. 17:4143–4148.2. Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011. 7:463–475.3. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001. 12:1135–1148.4. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.5. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010. 195:758–765.6. Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011. 196:W565–W572.7. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012. 13:34–43.8. Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013. 36:166–175.9. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005. 242:158–171.10. de Baere T. [Ablation of liver metastases by radiofrequency]. Cancer Radiother. 2012. 16:339–343.11. Dodd GD 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001. 177:777–782.12. Bangard C. [Radiofrequency of the liver - an update]. Rofo. 2011. 183:704–713.13. Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Radiofrequency ablation for viable hepatocellular carcinoma around retained iodized oil after transcatheter arterial chemoembolization: usefulness of biplane fluoroscopy plus ultrasound guidance. Korean J Radiol. 2012. 13:784–794.14. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001. 13:129–147.15. Widmann G, Bodner G, Bale R. Tumour ablation: technical aspects. Cancer Imaging. 2009. 9 Spec No A:S63–S67.16. McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010. 21:8 Suppl. S204–S213.17. Seong NJ, Yoon CJ, Kang SG, Chung JW, Kim HC, Park JH. Effects of arsenic trioxide on radiofrequency ablation of VX2 liver tumor: intraarterial versus intravenous administration. Korean J Radiol. 2012. 13:195–201.18. Lee ES, Lee JM, Kim WS, Choi SH, Joo I, Kim M, et al. Multiple-electrode radiofrequency ablations using Octopus® electrodes in an in vivo porcine liver model. Br J Radiol. 2012. 85:e609–e615.19. Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007. 42:163–171.20. Stoffner R, Kremser C, Schullian P, Haidu M, Widmann G, Bale RJ. Multipolar radiofrequency ablation using 4-6 applicators simultaneously: a study in the ex vivo bovine liver. Eur J Radiol. 2012. 81:2568–2575.21. Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007. 42:676–683.22. Clasen S, Schmidt D, Boss A, Dietz K, Kröber SM, Claussen CD, et al. Multipolar radiofrequency ablation with internally cooled electrodes: experimental study in ex vivo bovine liver with mathematic modeling. Radiology. 2006. 238:881–890.23. Lee JM, Han JK, Lee JY, Kim SH, Choi JY, Lee MW, et al. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol. 2006. 7:106–117.24. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.25. Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg. 2008. 195:508–520.26. Lin SM. Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers. Chang Gung Med J. 2009. 32:22–32.27. Ritz JP, Lehmann KS, Reissfelder C, Albrecht T, Frericks B, Zurbuchen U, et al. Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Colorectal Dis. 2006. 21:25–32.28. Clasen S, Rempp H, Schmidt D, Schraml C, Hoffmann R, Claussen CD, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol. 2012. 81:111–113.29. Lee FT Jr, Haemmerich D, Wright AS, Mahvi DM, Sampson LA, Webster JG. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003. 14:1437–1442.30. Lee IJ, Kim YI, Kim KW, Kim DH, Ryoo I, Lee MW, et al. Radiofrequency ablation combined with transcatheter arterial embolisation in rabbit liver: investigation of the ablation zone according to the time interval between the two therapies. Br J Radiol. 2012. 85:e987–e994.31. Lee JM, Han JK, Kim SH, Choi SH, An SK, Han CJ, et al. Bipolar radiofrequency ablation using wet-cooled electrodes: an in vitro experimental study in bovine liver. AJR Am J Roentgenol. 2005. 184:391–397.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monopolar Radiofrequency Ablation Using a Dual-Switching System and a Separable Clustered Electrode: Evaluation of the In Vivo Efficiency
- No-Touch Radiofrequency Ablation: A Comparison of Switching Bipolar and Switching Monopolar Ablation in Ex Vivo Bovine Liver
- Ablative Outcomes of Various Energy Modes for No-Touch and Peripheral Tumor-Puncturing Radiofrequency Ablation: An Ex Vivo Simulation Study
- Percutaneous Dual-Switching Monopolar Radiofrequency Ablation Using a Separable Clustered Electrode: A Preliminary Study
- Radiofrequency Ablation Using a Separable Clustered Electrode for the Treatment of Hepatocellular Carcinomas: A Randomized Controlled Trial of a DualSwitching Monopolar Mode Versus a Single-Switching Monopolar Mode