Korean J Ophthalmol.
2013 Aug;27(4):299-303. 10.3341/kjo.2013.27.4.299.
Regression of Iris Neovascularization after Subconjunctival Injection of Bevacizumab
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea.
- 2Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. twkim7@snu.ac.kr
- KMID: 1705429
- DOI: http://doi.org/10.3341/kjo.2013.27.4.299
Abstract
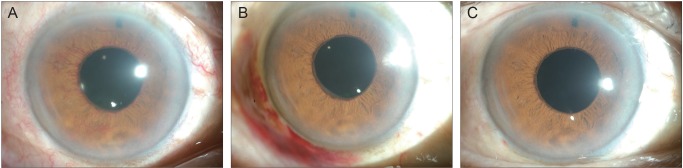
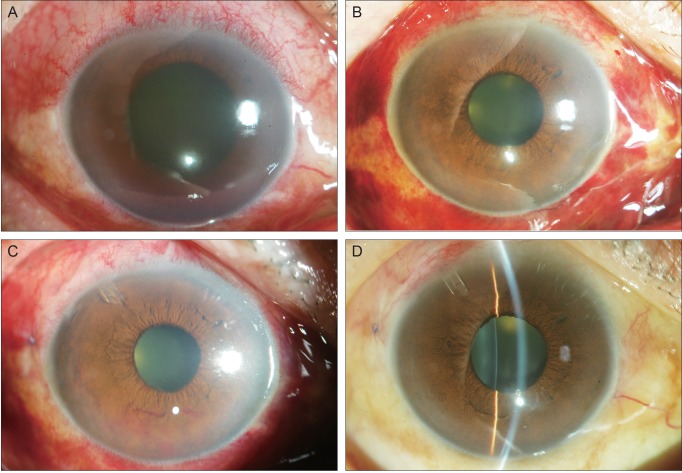
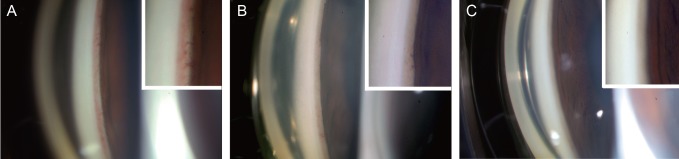
- To describe three cases of neovascular glaucoma (NVG) where iris or angle neovascularization regressed remarkably after subconjunctival bevacizumab injections used as the initial treatment before pan retinal photocoagulation (PRP) and/or filtering surgery. Three consecutive NVG patients whose intraocular pressure (IOP) was not controlled with maximal medication were offered an off-label subconjunctival injection of bevacizumab (2.5-3.75 mg/0.1-0.15 mL, Avastin). Bevacizumab was injected into the subconjunctival space close to the corneal limbus in two or three quadrants using a 26-gauge needle. Serial anterior segment photographs were taken before and after the injection. Following subconjunctival injection of bevacizumab, iris or angle neovascularization regressed rapidly within several days. Such regression was accompanied by lowering of IOP in all three cases. The patients underwent subsequent PRP and/or filtering surgery, and the IOP was further stabilized. Our cases demonstrate that subconjunctival bevacizumab injection can be potentially useful as an initial treatment in NVG patients before laser or surgical treatment.
MeSH Terms
-
Adult
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal, Humanized/*administration & dosage
*Conjunctiva
Glaucoma, Neovascular/*drug therapy
Humans
Injections, Intraocular
Iris Diseases/*drug therapy
Male
Middle Aged
Treatment Outcome
Angiogenesis Inhibitors
Antibodies, Monoclonal, Humanized
Figure
Reference
-
1. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–354. PMID: 16508438.
Article2. Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006; 142:1054–1056. PMID: 17157590.
Article3. Vatavuk Z, Bencic G, Mandic Z. Intravitreal bevacizumab for neovascular glaucoma following central retinal artery occlusion. Eur J Ophthalmol. 2007; 17:269–271. PMID: 17415704.
Article4. Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007; 16:437–439. PMID: 17700285.
Article5. Moraczewski AL, Lee RK, Palmberg PF, et al. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol. 2009; 93:589–593. PMID: 19074917.
Article6. Eid TM, Radwan A, el-Manawy W, el-Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Can J Ophthalmol. 2009; 44:451–456. PMID: 19606170.
Article7. Grisanti S, Biester S, Peters S, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006; 142:158–160. PMID: 16815268.
Article8. Shin JP, Lee JW, Sohn BJ, et al. In vivo corneal endothelial safety of intracameral bevacizumab and effect in neovascular glaucoma combined with Ahmed valve implantation. J Glaucoma. 2009; 18:589–594. PMID: 19826387.
Article9. Ip MS, Scott IU, Brown GC, et al. Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2008; 115:1837–1846. PMID: 18929163.10. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–1349. PMID: 16854824.
Article11. Komori S, Sawada A, Oguni T, et al. Case of endophthalmitis following intravitreal injections of bevacizumab. Clin Ophthalmol. 2010; 4:773–775. PMID: 20689793.12. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011; 95:1111–1114. PMID: 20702430.
Article13. Kim MJ, Han ES, Kim J, Kim TW. Aqueous humor concentration of bevacizumab after subconjunctival injection in rabbit. J Ocul Pharmacol Ther. 2010; 26:49–53. PMID: 20148650.
Article14. Chu HS, Hu FR, Yang CM, et al. Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea. 2011; 30:60–66. PMID: 20847676.
Article15. Symes RJ, Poole TR. Corneal graft surgery combined with subconjunctival bevacizumab (Avastin). Cornea. 2010; 29:691–693. PMID: 20458243.
Article16. Yazdani S, Hendi K, Pakravan M, et al. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009; 18:632–637. PMID: 19826393.17. Kuo JZ, Ong FS, Yeung L, et al. Predictive factors for visual outcome to intravitreal bevacizumab in young Chinese patients with myopic choroidal neovascularization. Retina. 2011; 31:1835–1840. PMID: 21878845.
Article18. Marey HM, Ellakwa AF. Intravitreal bevacizumab alone or combined with triamcinolone acetonide as the primary treatment for diabetic macular edema. Clin Ophthalmol. 2011; 5:1011–1016. PMID: 21845026.
Article19. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908. PMID: 21526923.
Article20. Kim TW, Lindsey JD, Aihara M, et al. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci. 2002; 43:1809–1816. PMID: 12036983.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bi-weekly Subconjunctival Injection of Bevacizumab for Corneal Neovascularization after Burn Injury
- Acute Visual Loss after Intravitreal Bevacizumab Injection in a Patient with Ocular Ischemic Syndrome
- A Case of Ocular Benign Lymphoid Hyperplasia Treated with Bevacizumab Injection
- Intraviteal Bevacizumab (Avastin(R)) Injection for the Treatment of Early-Stage Neovascular Glaucoma
- A Case of Iris Neovascularization in Severe Fungal Keratitis Treated with Intracameral Bevacizumab