J Clin Neurol.
2006 Jun;2(2):83-91. 10.3988/jcn.2006.2.2.83.
Syndromic Approach to Parkinson's Disease: Role of Functional Imaging
- Affiliations
-
- 1Department of Medicine/Neurology, Vancouver Hospital and Health Sciences Centre, University of British Columbia, Vancouver, Canada. cslee@interchange.ubc.ca
- 2Department of Neurology, College of Medicine, University of Ulsan, Seoul, Korea.
- 3Department of Nuclear Medicine, College of Medicine, University of Ulsan, Seoul, Korea.
- KMID: 1700740
- DOI: http://doi.org/10.3988/jcn.2006.2.2.83
Abstract
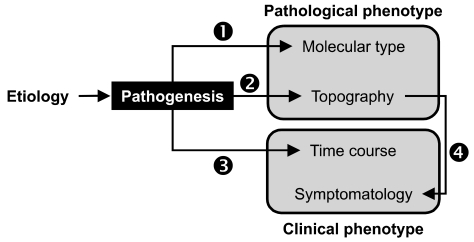
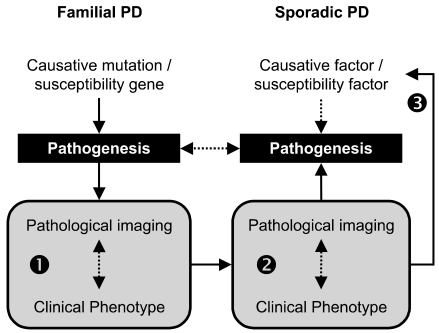
- Current evidence from monogenic Parkinson's disease (PD) supports the view that PD is a clinical syndrome, rather than a single disease entity, and that the heterogeneity of PD indeed reflects different pathogenesis. Recent developments in functional imaging have enabled the in vivo assessment of cellular and molecular pathology of PD with respect to temporal and topographical patterns. We propose that this new technology will be useful for linking monogenic and sporadic PD, and thus, for classifying PD based on the pathogenesis. It will be also useful in clinico-genetic studies exploring susceptibility factors and at-risk groups, which are important for neuroprotective treatment when it becomes available.
Keyword
MeSH Terms
Figure
Reference
-
1. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997; 276:2045–2047. PMID: 9197268.2. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840. PMID: 9278044.3. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988; 51:745–752. PMID: 2841426.
Article4. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993; 50:140–148. PMID: 8431132.
Article5. Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006; 7:306–318. PMID: 16543934.
Article6. Wszolek ZK, Pfeiffer RF, Tsuboi Y, Uitti RJ, McComb RD, Stoessl AJ, et al. Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology. 2004; 62:1619–1622. PMID: 15136696.
Article7. Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004; 44:601–607. PMID: 15541309.
Article8. Pramstaller PP, Schlossmacher MG, Jacques TS, Scaravilli F, Eskelson C, Pepivani I, et al. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005; 58:411–422. PMID: 16130111.9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184. PMID: 1564476.
Article10. Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism--a prospective study. Can J Neurol Sci. 1991; 18:275–278. PMID: 1913360.11. Singleton A, Gwinn-Hardy K. Parkinson's disease and dementia with Lewy bodies: a difference in dose? Lancet. 2004; 364:1105–1107. PMID: 15451205.
Article12. Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006; 354:424–425. PMID: 16436782.13. Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med. 2006; 354:422–423. PMID: 16436781.14. Tomiyama H, Li Y, Funayama M, Hasegawa K, Yoshino H, Kubo SI, et al. Clinicogenetic study of mutations in LRRK2 exon 41 in Parkinson's disease patients from 18 countries. Mov Disord. 2006; 21:1102–1108. Apr 18 [Epub]. PMID: 16622854.15. Gosal D, Ross OA, Wiley J, Irvine GB, Johnston JA, Toft M, et al. Clinical traits of LRRK2-associated Parkinson's disease in Ireland: a link between familial and idiopathic PD. Parkinsonism Relat Disord. 2005; 11:349–352. PMID: 16102999.
Article16. Fishman PS, Oyler GA. Significance of the parkin gene and protein in understanding Parkinson's disease. Curr Neurol Neurosci Rep. 2002; 2:296–302. PMID: 12044248.
Article17. Calne DB. Is "Parkinson's disease" one disease? J Neurol Neurosurg Psychiatry. 1989; 2(Suppl):18–21. PMID: 2666575.
Article18. Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003; 991:1–14. PMID: 12846969.
Article19. Klein C. Implications of genetics on the diagnosis and care of patients with Parkinson disease. Arch Neurol. 2006; 63:328–334. PMID: 16533959.
Article20. Golbe LI, Di Iorio G, Bonavita V, Miller DC, Duvoisin RC. A large kindred with autosomal dominant Parkinson's disease. Ann Neurol. 1990; 27:276–282. PMID: 2158268.
Article21. Welch K, Yuan J. Alpha-synuclein oligomerization: a role for lipids? Trends Neurosci. 2003; 26:517–519. PMID: 14522142.22. West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005; 102:16842–16847. PMID: 16269541.23. Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006; 15:223–232. PMID: 16321986.
Article24. Shen J, Cookson MR. Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron. 2004; 43:301–304. PMID: 15294138.25. Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005; 74:29–52. PMID: 15952880.
Article26. Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990; 40:1529–1534. PMID: 2215943.27. Graham JM, Sagar HJ. A data-driven approach to the study of heterogeneity in idiopathic Parkinson's disease: identification of three distinct subtypes. Mov Disord. 1999; 14:10–20. PMID: 9918339.28. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005; 76:343–348. PMID: 15716523.
Article29. Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004; 55:306–319. PMID: 14991808.
Article30. Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005; 57:168–175. PMID: 15668962.
Article31. Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005; 65:1716–1722. PMID: 16344512.
Article32. Hilker R, Klein C, Ghaemi M, Kis B, Strotmann T, Ozelius LJ, et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the parkin gene. Ann Neurol. 2001; 49:367–376. PMID: 11261512.33. Khan NL, Brooks DJ, Pavese N, Sweeney MG, Wood NW, Lees AJ, et al. Progression of nigrostriatal dysfunction in a parkin kindred: an [18F]dopa PET and clinical study. Brain. 2002; 125:2248–2256. PMID: 12244082.34. Thobois S, Ribeiro MJ, Lohmann E, Durr A, Pollak P, Rascol O, et al. Young-onset Parkinson disease with and without parkin gene mutations: a fluorodopa F 18 positron emission tomography study. Arch Neurol. 2003; 60:713–718. PMID: 12756135.35. Khan NL, Scherfler C, Graham E, Bhatia KP, Quinn N, Lees AJ, et al. Dopaminergic dysfunction in unrelated, asymptomatic carriers of a single parkin mutation. Neurology. 2005; 64:134–136. PMID: 15642918.
Article36. Khan NL, Valente EM, Bentivoglio AR, Wood NW, Albanese A, Brooks DJ, et al. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann Neurol. 2002; 52:849–853. PMID: 12447943.37. Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005; 64:1958–1960. PMID: 15955954.
Article38. Hering R, Strauss KM, Tao X, Bauer A, Woitalla D, Mietz EM, et al. Novel homozygous p.E64D mutation in DJ1 in early onset Parkinson disease (PARK7). Hum Mutat. 2004; 24:321–329. PMID: 15365989.
Article39. Paisan-Ruiz C, Saenz A, Lopez de Munain A, Marti I, Martinez Gil A, Marti-Masso JF, et al. Familial Parkinson's disease: clinical and genetic analysis of four Basque families. Ann Neurol. 2005; 57:365–372. PMID: 15732106.40. Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005; 76:672–680. PMID: 15726496.
Article41. Hernandez DG, Paisan-Ruiz C, McInerney-Leo A, Jain S, Meyer-Lindenberg A, Evans EW, et al. Clinical and positron emission tomography of Parkinson's disease caused by LRRK2. Ann Neurol. 2005; 57:453–456. PMID: 15732108.42. Adams JR, van Netten H, Schulzer M, Mak E, McKenzie J, Strongosky A, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain. 2005; 128:2777–2785. PMID: 16081470.
Article43. Isaias IU, Benti R, Goldwurm S, Zini M, Cilia R, Gerundini P, et al. Striatal dopamine transporter binding in Parkinson's disease associated with the LRRK2 Gly2019Ser mutation. Mov Disord. 2006; 21:1144–1147. May 2 [Epub]. PMID: 16671078.44. Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol. 1983; 13:243–248. PMID: 6847136.
Article45. Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimers disease. J Neurol Neurosurg Psychiatry. 1985; 48:413–421. PMID: 3998751.
Article46. Dubois B, Danze F, Pillon B, Cusimano G, Lhermitte F, Agid Y. Cholinergic-dependent cognitive deficits in Parkinson's disease. Ann Neurol. 1987; 22:26–30. PMID: 3631918.
Article47. Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997; 349:1805–1809. PMID: 9269216.
Article48. Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, et al. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease. Neurology. 1999; 52:691–699. PMID: 10078712.
Article49. Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, et al. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer's disease: a positron emission tomography study. Ann Neurol. 2000; 48:194–200. PMID: 10939570.
Article50. Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy. Ann Neurol. 1999; 46:62–69. PMID: 10401781.
Article51. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003; 60:1745–1748. PMID: 14676050.52. Herholz K, Weisenbach S, Kalbe E, Diederich NJ, Heiss WD. Cerebral acetylcholine esterase activity in mild cognitive impairment. Neuroreport. 2005; 16:1431–1434. PMID: 16110265.
Article53. Shinotoh H, Aotsuka A, Fukushi K, Nagatsuka S, Tanaka N, Ota T, et al. Effect of donepezil on brain acetylcholinesterase activity in patients with AD measured by PET. Neurology. 2001; 56:408–410. PMID: 11171913.
Article54. Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996; 40:399–410. PMID: 8797529.
Article55. Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Mathis CA. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 2001; 69:1471–1484. PMID: 11554609.
Article56. Heilig CW, Knopman DS, Mastri AR, Frey W 2nd. Dementia without Alzheimer pathology. Neurology. 1985; 35:762–765. PMID: 2986048.
Article57. Boller F, Mizutani T, Roessmann U, Gambetti P. Parkinson disease, dementia, and Alzheimer disease: clinicopathological correlations. Ann Neurol. 1980; 7:329–335. PMID: 7377758.
Article58. Jellinger K. Alzheimer pathology in Parkinson's disease. Neurology. 1989; 39:874–875. PMID: 2725894.
Article59. Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm. 2002; 109:329–339. PMID: 11956955.
Article60. Charbonneau P, Syrota A, Crouzel C, Valois JM, Prenant C, Crouzel M. Peripheral-type benzodiazepine receptors in the living heart characterized by positron emission tomography. Circulation. 1986; 73:476–483. PMID: 3004781.
Article61. McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988; 24:574–576. PMID: 3239957.
Article62. McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995; 21:195–218. PMID: 8866675.
Article63. Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006; 21:404–412. PMID: 16182554.64. Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003; 299:256–259. PMID: 12446870.65. Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004; 101:9103–9108. PMID: 15181200.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Team Approach Rehabilitation Program on Balance, Gait, and Muscle Strength of Lower Extremities for Elderly Patients with Parkinson's Disease
- Imaging of Dopaminergic System in Movement Disorders
- Practical Approach for the Clinical Use of Dopamine Transporter Imaging
- Diagnosis and Treatment of Parkinson's Disease
- MRI Findings in Parkinson’s Disease: Radiologic Assessment of Nigrostriatal Degeneration