J Korean Soc Magn Reson Med.
2014 Mar;18(1):7-16. 10.13104/jksmrm.2014.18.1.7.
First-pass Stress Perfusion MR Imaging Findings of Apical Hypertrophic Cardiomyopathy: with Relation to LV Wall Thickness and Late Gadolinium-enhancement
- Affiliations
-
- 1Division of Cardiovascular Imaging, Department of Radiology, Seoul National University Bundang Hospital, Seoul, Korea. drejchun@hanmail.net
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seoul, Korea.
- KMID: 1683775
- DOI: http://doi.org/10.13104/jksmrm.2014.18.1.7
Abstract
- PURPOSE
To evaluate the prevalence and pattern of perfusion defect (PD) on first-pass stress perfusion MR imaging in relation with the degree of left ventricular hypertrophy (LVH) and late gadolinium-enhancement (LGE) in patients with apical hypertrophic cardiomyopathy (APH).
MATERIALS AND METHODS
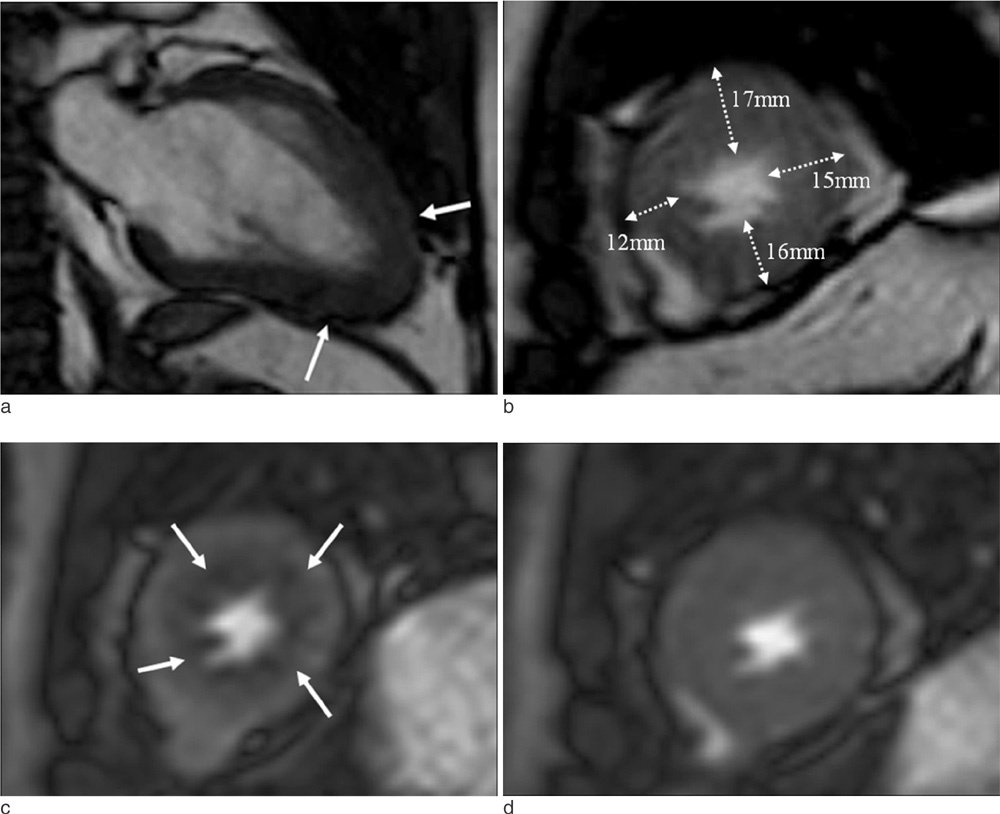
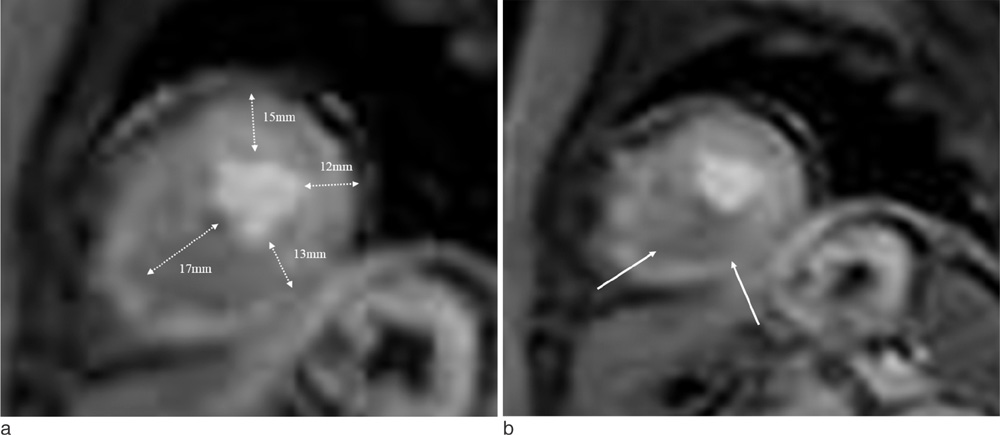
Cardiac MR imaging with first-pass stress perfusion, cine, and LGE sequence was performed in 26 patients with APH from January 2008 to December 2012. We analyzed a total of 416 segments for LV wall thickness on end-diastolic phase of cine images, and evaluated the number of hypertrophied segment and number of consecutive hypertrophied segment (NCH). We assessed the presence or absence of PD and LGE from all patients. If there was PD, we subdivided the pattern into sporadic (sporadic-PD) or ring (ring-PD). Using univariate logistic method, we obtained the independent predictor for presence of overall PD and ring-PD.
RESULTS
PD on stress perfusion MRI was observed in 20 patients (76.9%), 12 of them (60%) showed ring-PD. Maximal LV wall thickness and number of hypertrophied segment were independent predictors for overall PD (all, p < 0.05). NCH with more than 3 segments was an additional independent factor for ring-PD. However, LGE was not statistically related with PD in patients with APH.
CONCLUSION
About three quarters of the patients with APH showed PD, most of them represented as ring-PD. LVH degree or distribution was related with pattern of PD, however, LGE was not related with PD. Therefore, the clinical significance of PD in the patients with APH seems to be different from those with non-APH, and further comparison study between the two groups should be carried out.
MeSH Terms
Figure
Reference
-
1. Maron BJ, McKenna WJ, Danielson GK, et al. American college of cardiology/european society of cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the american college of cardiology foundation task force on clinical expert consensus documents and the european society of cardiology committee for practice guidelines. J Am Coll Cardiol. 2003; 42:1687–1713.2. Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasono-cardiotomographic study. Jpn Heart J. 1976; 17:611–629.3. Chun EJ, Choi SI, Jin KN, et al. Hypertrophic cardiomyopathy: Assessment with MR imaging and multidetector CT. Radiographics. 2010; 30:1309–1328.4. Okishige K, Sasano T, Yano K, Azegami K, Suzuki K, Itoh K. Serious arrhythmias in patients with apical hypertrophic cardiomyopathy. Intern Med. 2001; 40:396–402.5. Kusukawa J, Suwa M, Nakayama Y, et al. Advanced sequelae of apical hypertrophic cardiomyopathy: report of two cases with wall motion abnormalities. J Cardiol. 1988; 18:259–269.6. Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med. 2004; 350:1320–1327.7. Spirito P, Bellone P. Natural history of hypertrophic cardiomyopathy. Br Heart J. 1994; 72:6 Suppl. S10–S12.8. Sipola P, Lauerma K, Husso-Saastamoinen M, et al. First-pass MR imaging in the assessment of perfusion impairment in patients with hypertrophic cardiomyopathy and the Asp175Asn mutation of the alpha-tropomyosin gene. Radiology. 2003; 226:129–137.9. Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009; 2:412–424.10. Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004; 90:645–649.11. Writing Committee Members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:e240–e327.12. Sorajja P, Nishimura RA, Gersh BJ, et al. Outcome of mildly symptomatic or asymptomatic obstructive hypertrophic cardiomyopathya long-term follow-up study. J Am Coll Cardiol. 2009; 54:234–241.13. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002; 105:539–542.14. Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008; 29:480–489.15. Chung SY, Lee KY, Chun EJ, et al. Comparison of stress perfusion mri and spect for detection of myocardial ischemia in patients with angiographically proven three-vessel coronary artery disease. AJR Am J Roentgenol. 2010; 195:356–362.16. Harrigan CJ, Peters DC, Gibson CM, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular mr imaging. Radiology. 2011; 258:128–133.17. Sakamoto T, Amano K, Hada Y, et al. Asymmetric apical hypertrophy: ten years experience. Postgrad Med J. 1986; 62:567–570.18. Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 39:638–645.19. Matsubara K, Nakamura T, Kuribayashi T, Azuma A, Nakagawa M. Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 42:288–295.20. Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992; 86:38–46.21. Krams R, Kofflard M, Duncker D, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998; 97:230–233.22. von Dohlen TW, Prisant LM, Frank MJ. Significance of positive or negative thallium-201 scintigraphy in hypertrophic cardiomyopathy. Am J Cardiol. 1989; 64:498–503.23. Cannon RO 3rd, Dilsizian V, O'Gara PT, et al. Myocardial metabolic, hemodynamic, and electrocardiographic significance of reversible thallium-201 abnormalities in hypertrophic cardiomyopathy. Circulation. 1991; 83:1660–1667.24. Lee KH, Jang HJ, Lee SC, et al. Myocardial thallium defects in apical hypertrophic cardiomyopathy are associated with a benign prognosis. Int J Cardiovasc Imaging. 2003; 19:381–388.25. Panting JR, Gatehouse PD, Yang G-Z, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002; 346:1948–1953.26. Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart. 2007; 93:159–166.27. Yamada M, Elliott P, Kaski J, et al. Dipyridamole stress thallium-201 perfusion abnormalities in patients with hypertrophic cardiomyopathy. Relationship to clinical presentation and outcome. Eur Heart J. 1998; 19:500–507.28. Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993; 22:796–804.29. O'Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56:867–874.30. Cecchi F, Sgalambro A, Baldi M. Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009; 2:452–461.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Relation of Late Gadolinium Enhancement to Increased Ventricular Wall Stress in Dilated Cardiomyopathy
- Myocardial Fibrosis in Hypertrophic Cardiomyopathy Demonstrated by Integrated Cardiac F-18 FDG PET/MR
- A Case of Regressed Apical Hypertrophic Cardiomyopathy
- Apical Hypertrophic Cardiomyopathy with Apical Aneurysm and Thrombus Diagnosed by Contrast Echocardiography
- Dipyridamole Tl-201 SPECT in Hypertrophic Cardiomyopathy with Asymmetric Septal Hypertrophy:Characteristics of Perfusion Abnormality and Correlation with Clinical Parameters