J Korean Diabetes Assoc.
2007 May;31(3):230-235. 10.4093/jkda.2007.31.3.230.
Effects of Lovastatin on Free Fatty Acid Oxidation in Cultured L6 Rat Skeletal Muscle Cells
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Korea.
- KMID: 1523011
- DOI: http://doi.org/10.4093/jkda.2007.31.3.230
Abstract
-
BACKGROUND: Recent clinical studies suggest that statins improve insulin resistance and glucose metabolism in patients with metabolic syndrome and type 2 diabetes. To evaluate the possible mechanism of this action, we measured free fatty acid oxidation in cultured L6 rat skeletal muscle cell line.
METHODS
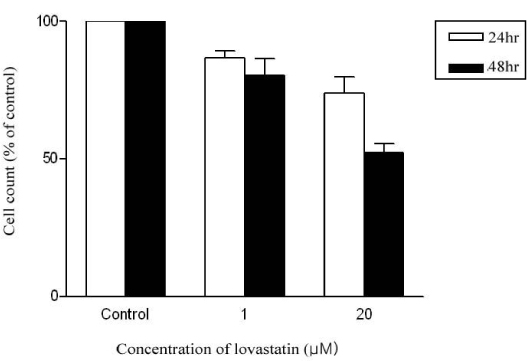
Cultured L6 myotubes were treated with or without lovastatin (1, 5, 20 micrometer) for 24 hours or 48 hours and palmitate oxidation was measured. We also measured protein concentration of the cells.
RESULTS
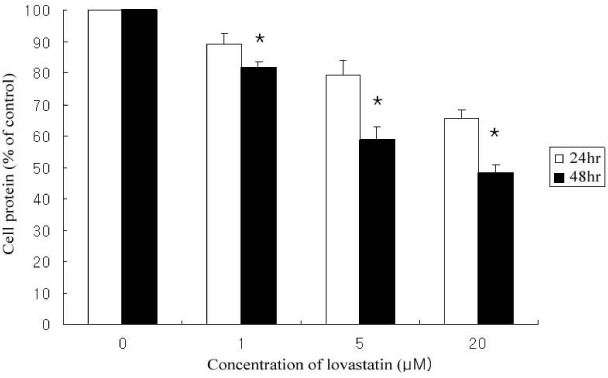
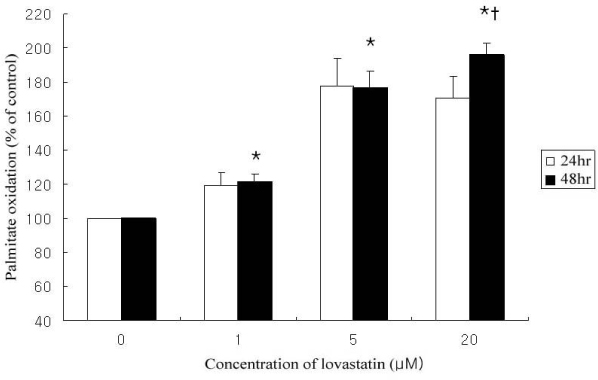
Lovastain increased palmitate oxidation in dose and time dependent manner in L6 myotubes (24 hr; 1 micrometer 119.2 +/- 11.9% of control, 5 micrometer 140.9 +/- 8.1%, 20 micrometer 150 +/- 5%, P = 0.05 vs control, respectively, 48 hr 1 micrometer 120.9 +/- 14.5%, 5 micrometer 176.6 +/- 28.2%, 20 micrometer 196.0 +/- 19.9%, P < 0.01 vs control, respectively). However, lovastatin decreased total cellular protein (24 hr: 1 micrometer 89.2 +/- 6.1% of control, 5 micrometer 79.3 +/- 7.6%, 20 micrometer 65.4 +/- 4.2%, P = 0.05 vs control, respectively, 48 hr: 1 micrometer 81.7 +/- 5.1%, 5 micrometer 58.6 +/- 11.9%, 20 micrometer 48.1 +/- 6.9%, P < 0.01 vs control, respectively).
CONCLUSION
Lovastatin increased skeletal muscle free fatty acid oxidation in L6 rat skeletal muscle cells. This would be one of the mechanisms which lovastatin improves insulin resistance.
Keyword
MeSH Terms
Figure
Reference
-
1. DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992. 15:318–368.2. Baron AD, Laakso M, Brechtel G, Edelman SV. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in non-insulin dependent diabetic subjects. J Clin Inves. 1991. 87(4):1186–1194.3. Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994. 93:2438–2446.4. Kelly DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J physiol. 1999. 277:E1130–E1141.5. Kelly DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994. 26:2349–2356.6. Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes. 2004. 53:542–548.7. Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002. 22:325–346.8. Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001. 50:2337–2343.9. Mingrone G, DeGaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M, Gasbarrini G. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997. 40:599–605.10. Guclu F, Ozmen B, Hekimsoy Z, Kirmaz C. Effects of a statin group drug, pravastatin, on the insulin resistance in patients with metabolic syndrome. Biomed Pharmacother. 2004. 58:614–618.Goodpaster BH., Katsiaras A., Kelly DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003. 52(9):2191–2197.12. Deedwania PC, Hunninghake DB, Bays H. Effects of lipid-altering treatment in diabetes mellitus and the metabolic syndrome. Am J Cardiol. 2004. 93(11A):18C–26C.13. Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, Varricchio M. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis. 2000. 150:121–127.14. Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001. 103(3):357–362.15. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.16. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endo Rev. 2002. 23:201–229.17. Carmena R, Betteridge DJ. Statins and diabetes. Semin Vasc Med. 2004. 4:321–332.18. Miyazaki Y, Glass L, Triplitt C, Matsuda M, Cusi K, Mahankali A, Mahankali S, Mandarino LJ, DeFronzo RA. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in type II diabetic patients. Diabetologia. 2001. 44:2210–2219.19. Gadbut AP, Caruso AP, Galper JB. Differential sensitivity of C2-C12 striated muscle cells to lovasatin and pravastatin. J Mol Cell Cardiol. 1995. 27:2397–2402.20. Masters BA, Palmoski MJ, Flint OP, Gregg RE, Wang-Iverson D, Durham SK. In vitro mytoxicity of the 3-hydroxy-3-methylglucaryl Coenzyme A reductase inhibitors, pravastatin, lovastatin and simvastatin using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol. 1995. 131:163–174.21. Funatsu T, Kakuta H, Takasu T, Miyata K. Atorvastatin increases hepatic fatty acid beta-oxidation in sucrose fed rats: comparison with an MTP inhibitor. Eur J Pharmacol. 2002. 455:161–167.22. Fukami M, Maeda N, Fukushige J, Kogure Y, Shimada Y, Ogawa T, Tsujita Y. Effects of HMG-CoA reductase inhibitors on skeletal muscles of rabbits. Res Exp Med. 1993. 193:263–273.23. El-Ani D, Zimlichman R. Simvastatin induces apoptosis of cultured rat cardiomyocytes. J Basic Clin Physiol Pharmacol. 2001. 12(4):325–338.24. Kaufmann P, Torok M, Zahno A, Waldhauser KM, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006. 63:2415–2425.25. Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006. 119:400–409.26. Hamilton JA, Guo W, Kamp F. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem. 2002. 239(1-2):17–23.27. Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acd-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab. 2002. 87(1):226–234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of lovastatin on free fatty acid oxidation in human skeletal muscle cells
- What is the Key Step in Muscle Fatty Acid Oxidation after Change of Plasma Free Fatty Acids Level in Rats?
- Effects of Free Fatty Acid on Insulin Secretion in Cultured Rat Pancreatic Islets
- Increase in Fatty Acid Oxidation by AICAR: the Role of p38 MAPK
- Antiproliferative Effect of Lovastatin on Vascular Smooth Muscle Cell