Ann Clin Microbiol.
2014 Mar;17(1):23-27. 10.5145/ACM.2014.17.1.23.
Postsurgical Wound Infection Caused by Mycobacterium conceptionense Identified by Sequencing of 16S rRNA, hsp65, and rpoB Genes in an Immunocompetent Patient
- Affiliations
-
- 1Department of Laboratory Medicine, Inje University College of Medicine, Busan, Korea.
- 2Paik Institute for Clinical Research, Inje University College of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 4Department of Dental Hygiene College of Medical and Life Science, Shilla University, Busan, Korea.
- 5Department of Laboratory Medicine, Korean Institute for Tuberculosis, Osong, Korea.
- 6Department of Laboratory Medicine, Pusan National University School of Medicine, Yangsan, Korea. cchl@pusan.ac.kr
- KMID: 1518101
- DOI: http://doi.org/10.5145/ACM.2014.17.1.23
Abstract
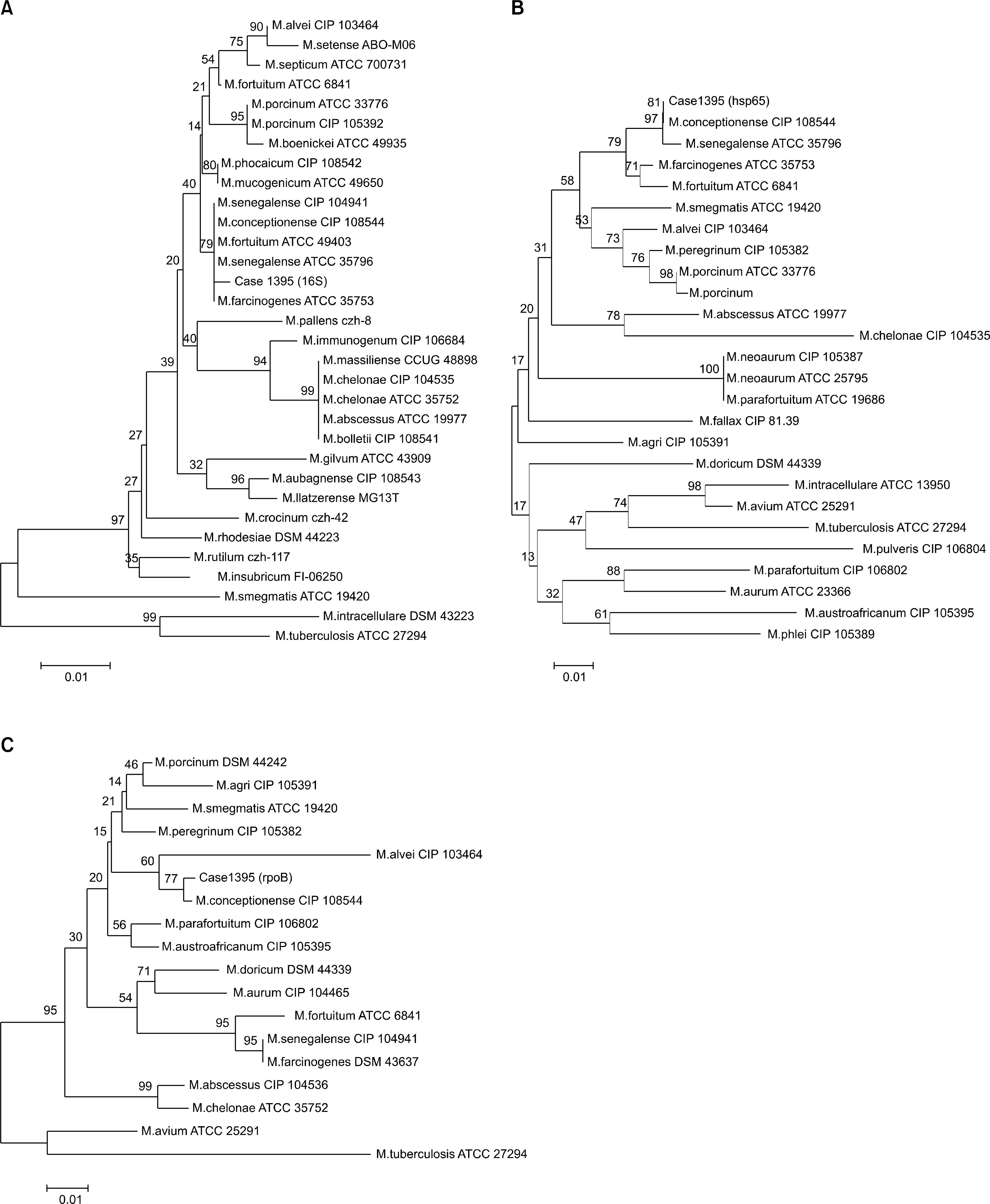
- Rapidly growing mycobacteria are ubiquitous in the environment and are increasingly being recognized as opportunistic pathogens. Recently, a new species, Mycobacteium conceptionense, has been validated from the Mycobacterium fortuitum third biovariant complex by molecular analysis. However, there are few reports, and postsurgical wound infection by this species is rare. We report a case of postsurgical wound infection caused by M. conceptionense in an immunocompetent patient that was identified by a sequencing analysis of 16S rRNA, hps65, and rpoB genes.
Figure
Reference
-
1.Colombo RE., Olivier KN. Diagnosis and treatment of infections caused by rapidly growing mycobacteria. Semin Respir Crit Care Med. 2008. 29:577–88.
Article2.Adékambi T., Berger P., Raoult D., Drancourt M. rpoB gene sequence-based characterization of emerging nontuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol. 2006. 56:133–43.3.Adékambi T., Drancourt M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int J Syst Evol Microbiol. 2004. 54:2095–105.4.Schinsky MF., Morey RE., Steigerwalt AG., Douglas MP., Wilson RW., Floyd MM, et al. Taxonomic variation in the Mycobacterium fortuitum third biovariant complex: description of Mycobacterium boenickei sp. nov., Mycobacterium houstonense sp. nov., Mycobacterium neworleansense sp. nov. and Mycobacterium brisbanense sp. nov. and recognition of Mycobacterium porcinum from human clinical isolates. Int J Syst Evol Microbiol. 2004. 54:1653–67.5.Adékambi T., Stein A., Carvajal J., Raoult D., Drancourt M. Descri-ption of Mycobacterium conceptionense sp. nov., a Mycobacterium fortuitum group organism isolated from a posttraumatic osteitis inflammation. J Clin Microbiol. 2006. 44:1268–73.6.Liao CH., Lai CC., Huang YT., Chou CH., Hsu HL., Hsueh PR. Subcutaneous abscess caused by Mycobacterium conceptionense in an immunocompetent patient. J Infect. 2009. 58:308–9.7.Thibeaut S., Levy PY., Pelletier ML., Drancourt M. Mycobacterium conceptionense infection after breast implant surgery, France. Emerg Infect Dis. 2010. 16:1180–1.8.Kim JH., Lee JY., Kim HR., Heo KW., Park SK., Lee JN, et al. Acute lymphadenitis with cellulitis caused by Staphylococcus lugdunensis. Korean J Lab Med. 2008. 28:196–200.9.Shin JH., Lee EJ., Lee HR., Ryu SM., Kim HR., Chang CL, et al. Prevalence of nontuberculous mycobacteria in a hospital environment. J Hosp Infect. 2007. 65:143–8.
Article10.Ringuet H., Akoua-Koffi C., Honore S., Varnerot A., Vincent V., Berche P, et al. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol. 1999. 37:852–7.11.Adékambi T., Drancourt M., Raoult D. The rpoB gene as a tool for clinical microbiologists. Trends Microbiol. 2009. 17:37–45.12.Clinical and Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. Document MM18-A. Wayne, PA; Clinical and Laboratory Standards Institute. 2010.13.Brown-Elliott BA., Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002. 15:716–46.
Article14.Murillo J., Torres J., Bofill L., Ríos-Fabra A., Irausquin E., Istúriz R, et al. Skin and wound infection by rapidly growing mycobacteria: an unexpected complication of liposuction and liposculpture. The Venezuelan Collaborative Infectious and Tropical Diseases Study Group. Arch Dermatol. 2000. 136:1347–52.15.Rodrigues C., Mehta A., Jha U., Bharucha M., Dastur FD., Udwadia TE. Nosocomial Mycobacterium chelonac infection in laparoscopic surgery. Infect Control Hosp Epidemiol. 2001. 22:474–5.16.Thami GP., Kaur S., Chander J., Attri AK. Post surgical atypical mycobacterial infection due to Mycobacterium fortuitum. J Infect. 2002. 45:210–11.17.Kalita JB., Rahman H., Baruah KC. Delayed postoperative wound infections due to nontuberculous mycobacterium. Indian J Med Res. 2005. 122:535–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Nontuberculous Mycobacteria by Sequence Analysis of the 16S Ribosomal RNA, the Heat-shock Protein 65 and the RNA Polymerase beta-Subunit Genes
- Five Rare Non-Tuberculous Mycobacteria Species Isolated from Clinical Specimens
- Sternal Osteomyelitis Caused by Gordonia bronchialis after Open-Heart Surgery
- Pulmonary Infection Caused by Mycobacterium neoaurum: The First Case in Korea
- A Case of Mycobacterium marinum Infection Diagnosed by PCR Amplification and Direct Sequencing