Yonsei Med J.
2013 Mar;54(2):437-444. 10.3349/ymj.2013.54.2.437.
Rapamycin Inhibits Transforming Growth Factor beta1-Induced Fibrogenesis in Primary Human Lung Fibroblasts
- Affiliations
-
- 1Department of Respiratory and Critical Care Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China. daihuaping@sina.com
- 2Beijing Key Laboratory of Respiratory and Pulmonary Circulation Disorders, Beijing Institute of Respiratory Medicine, Beijing, China.
- 3Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
- KMID: 1503908
- DOI: http://doi.org/10.3349/ymj.2013.54.2.437
Abstract
- PURPOSE
The present study was designed to determine whether rapamycin could inhibit transforming growth factor beta1 (TGF-beta1)-induced fibrogenesis in primary lung fibroblasts, and whether the effect of inhibition would occur through the mammalian target of rapamycin (mTOR) and its downstream p70S6K pathway.
MATERIALS AND METHODS
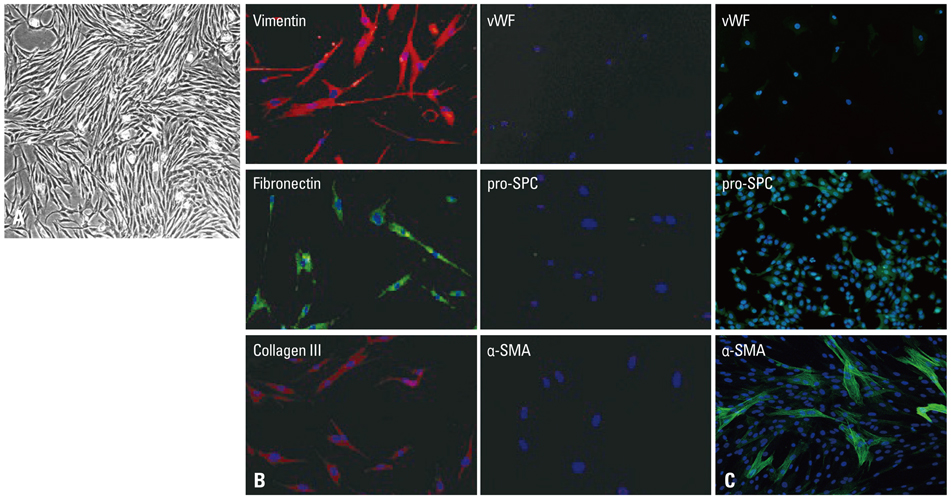
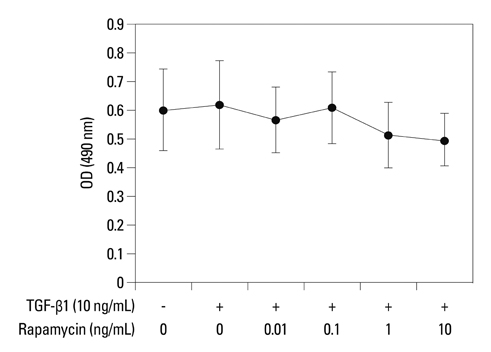
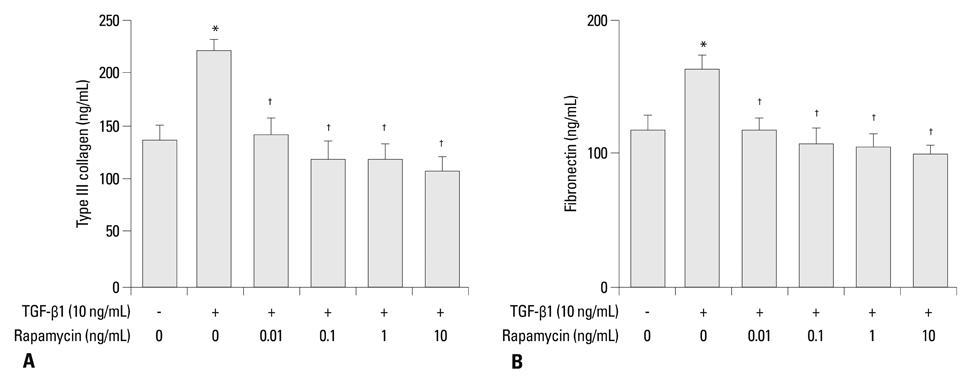
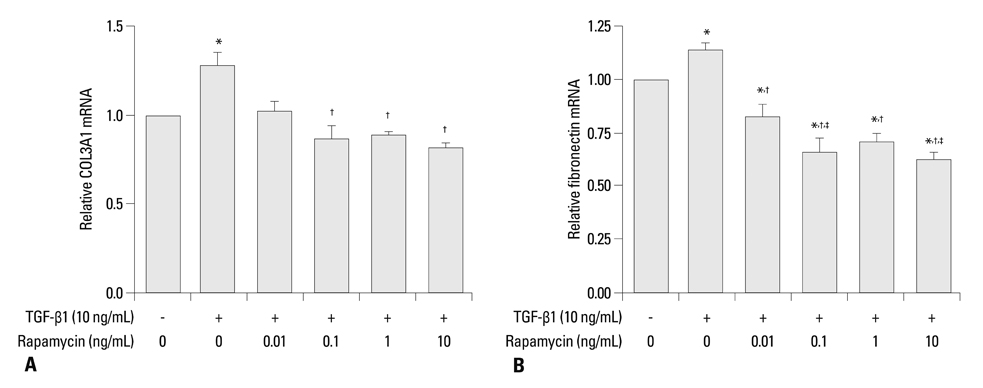
Primary normal human lung fibroblasts were obtained from histological normal lung tissue of 3 patients with primary spontaneous pneumothorax. Growth arrested, synchronized fibroblasts were treated with TGF-beta1 (10 ng/mL) and different concentrations of rapamycin (0.01, 0.1, 1, 10 ng/mL) for 24 h. We assessed m-TOR, p-mTOR, S6K1, p-S6K1 by Western blot analysis, detected type III collagen and fibronectin secreting by ELISA assay, and determined type III collagen and fibronectin mRNA levels by real-time PCR assay.
RESULTS
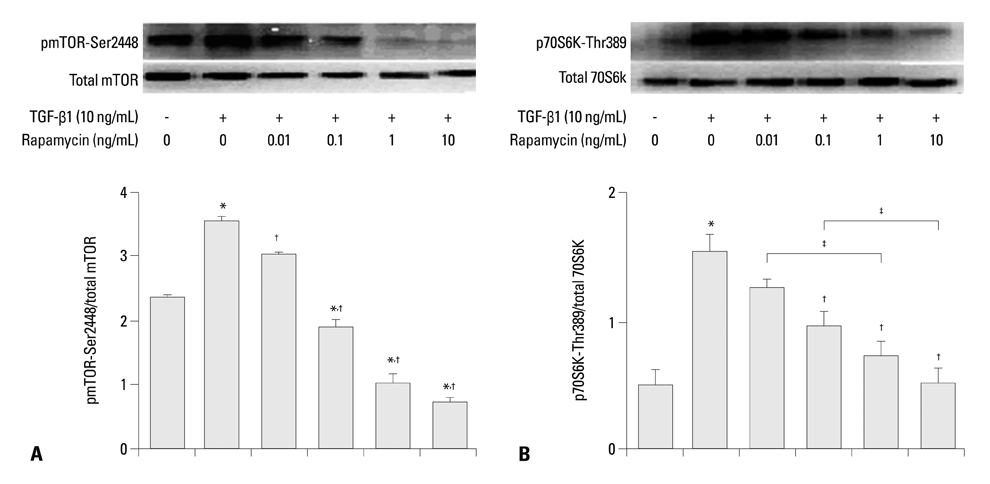
Rapamycin significantly reduced TGF-beta1-induced type III collagen and fibronectin levels, as well as type III collagen and fibronectin mRNA levels. Furthermore, we also found that TGF-beta1-induced mTOR and p70S6K phosphorylation were significantly down-regulated by rapamycin. The mTOR/p70S6K pathway was activated through the TGF-beta1-mediated fibrogenic response in primary human lung fibroblasts.
CONCLUSION
These results indicate that rapamycin effectively suppresses TGF-beta1-induced type III collagen and fibronectin levels in primary human lung fibroblasts partly through the mTOR/p70S6K pathway. Rapamycin has a potential value in the treatment of pulmonary fibrosis.
Keyword
MeSH Terms
-
Cells, Cultured
Collagen Type III/metabolism
Fibroblasts/*drug effects/metabolism/physiology
Fibronectins/metabolism
Humans
Lung/cytology/drug effects
Pulmonary Fibrosis/drug therapy
Signal Transduction/drug effects
Sirolimus/*pharmacology
TOR Serine-Threonine Kinases/metabolism/physiology
Transforming Growth Factor beta1/*antagonists & inhibitors/physiology
Collagen Type III
Fibronectins
Transforming Growth Factor beta1
TOR Serine-Threonine Kinases
Sirolimus
Figure
Reference
-
1. Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res. 2007. 24:819–841.
Article2. Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008. 40:2129–2140.
Article3. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004. 114:438–446.
Article4. Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007. 132:1311–1321.5. Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006. 3:377–382.
Article6. Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006. 81:1234–1248.
Article7. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.
Article8. Price KA, Azzoli CG, Krug LM, Pietanza MC, Rizvi NA, Pao W, et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol. 2010. 5:1623–1629.
Article9. Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, et al. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002. 19:1124–1127.
Article10. Bridle KR, Popa C, Morgan ML, Sobbe AL, Clouston AD, Fletcher LM, et al. Rapamycin inhibits hepatic fibrosis in rats by attenuating multiple profibrogenic pathways. Liver Transpl. 2009. 15:1315–1324.
Article11. Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, et al. Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009. 41:562–572.
Article12. Swaminathan S, Arbiser JL, Hiatt KM, High W, Abul-Ezz S, Horn TD, et al. Rapid improvement of nephrogenic systemic fibrosis with rapamycin therapy: possible role of phospho-70-ribosomal-S6 kinase. J Am Acad Dermatol. 2010. 62:343–345.
Article13. Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006. 281:33045–33052.
Article14. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997. 100:768–776.
Article15. Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004. 125:754–765.
Article16. Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009. 37(Pt 4):849–854.
Article17. Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991. 5:155–162.
Article18. Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991. 88:6642–6646.
Article19. Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, et al. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997. 150:981–991.20. Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax. 2001. 56:549–556.
Article21. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005. 166:1321–1332.
Article22. Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L525–L534.23. Yoshioka S, Mukae H, Ishii H, Kakugawa T, Ishimoto H, Sakamoto N, et al. Alpha-defensin enhances expression of HSP47 and collagen-1 in human lung fibroblasts. Life Sci. 2007. 80:1839–1845.
Article24. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003. 425:577–584.
Article25. Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005. 118(Pt 16):3573–3584.26. Lamouille S, Derynck R. Emergence of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin axis in transforming growth factor-β-induced epithelial-mesenchymal transition. Cells Tissues Organs. 2011. 193:8–22.
Article27. Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007. 82:381–388.
Article28. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001. 29:e45.
Article29. Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, et al. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am J Physiol. 1999. 276(5 Pt 1):L814–L824.30. Lock HR, Sacks SH, Robson MG. Rapamycin at subimmunosuppressive levels inhibits mesangial cell proliferation and extracellular matrix production. Am J Physiol Renal Physiol. 2007. 292:F76–F81.
Article31. Verrecchia F, Mauviel A. Control of connective tissue gene expression by TGF beta: role of Smad proteins in fibrosis. Curr Rheumatol Rep. 2002. 4:143–149.
Article32. Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002. 118:211–215.
Article33. Mariani TJ, Roby JD, Mecham RP, Parks WC, Crouch E, Pierce RA. Localization of type I procollagen gene expression in silica-induced granulomatous lung disease and implication of transforming growth factor-beta as a mediator of fibrosis. Am J Pathol. 1996. 148:151–164.34. Waerntges S, Klingel K, Weigert C, Fillon S, Buck M, Schleicher E, et al. Excessive transcription of the human serum and glucocorticoid dependent kinase hSGK1 in lung fibrosis. Cell Physiol Biochem. 2002. 12:135–142.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Reactive Oxygen Species in Transforming Growth Factor - beta1 - inuduced Fibronectin Secretion and alpha - Smooth Muscle Actin Expression in Human Lung Fibroblasts
- Transforming growth factor-beta Does Not Induce Endothelin-1 Secretion in Primary Cultured Human Tenon's Fibroblasts
- The Transforming Growth Factor-beta1 Expression in Normal Laryngeal Mucosa, Laryngeal Dysplasia and Laryngeal Carcinoma
- The effect of mineral trioxide aggregate on the production of growth factors and cytokine by human periodontal ligament fibroblasts
- Production Of Gm-Csf And Tgf-beta 1 In Irradiated Human Gingival Fibroblasts Cultured With Lipopolysaccharide