Korean J Ophthalmol.
2013 Feb;27(1):48-51. 10.3341/kjo.2013.27.1.48.
Effect of Heat Shock Protein 72 Expression on Etoposide-induced Cell Death of Rat Retinal Ganglion Cells
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ckee@skku.edu
- 2Center for Clinical Research, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1501796
- DOI: http://doi.org/10.3341/kjo.2013.27.1.48
Abstract
- PURPOSE
To assess whether the expression of heat shock protein 72 (Hsp72) protects rat retinal ganglion cells (RGC-5) from apoptotic cell death.
METHODS
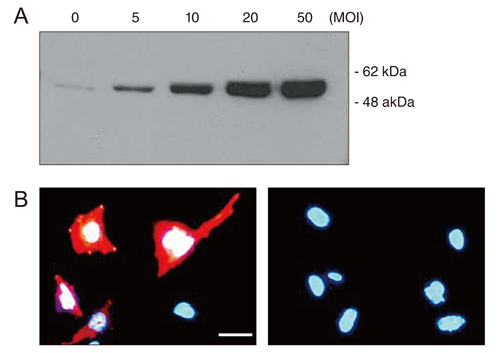
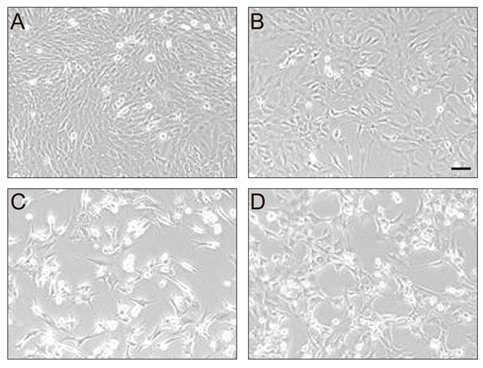
Hsp72 expression in RGC-5 cells transduced with replication-deficient recombinant adenovirus was analyzed by Western blot analysis and immunofluorescence. The effect of Hsp72 expression on etoposide-induced apoptotic cell death was examined by microscopic analysis and confirmed by cell proliferation assay.
RESULTS
Western blot analysis and immunofluorescence clearly showed adenovirus-mediated Hsp72 expression in RGC-5 cells. Treatment with etoposide resulted in the death of a proportion of the cells by apoptosis. However, this apoptotic cell death was significantly reduced in cells expressing Hsp72, with the reduction in cell death correlating to the level of Hsp72 expression.
CONCLUSIONS
Over-expression of Hsp72 alone is sufficient to rescue neuronal cells from apoptotic cell death, suggesting that fine-tuning its expression may be an effective neuroprotective approach in retinal degenerative disease.
MeSH Terms
-
Animals
Blotting, Western
Cell Death/*genetics
Cell Survival
Cells, Cultured
DNA/*genetics
Disease Models, Animal
Etoposide/toxicity
*Gene Expression Regulation
HSP72 Heat-Shock Proteins/biosynthesis/*genetics
Immunohistochemistry
Rats
Retinal Degeneration/*genetics/metabolism/pathology
Retinal Ganglion Cells/drug effects/*metabolism/pathology
HSP72 Heat-Shock Proteins
Etoposide
DNA
Figure
Reference
-
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996. 80:389–393.2. Shieds MB, Ritch R, Krupin T. Ritch R, Shields MB, Krupin T, editors. Classification of the glaucoma. The glaucomas. 1996. 2nd ed. St. Louis: Mosby;717–725.3. Shieds MB. Textbook of glaucoma. 1998. 4th ed. Baltimore: Williams & Wilkins;153–176.4. Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998. 80:183–201.5. Beere HM. "The stress of dying": the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004. 117(Pt 13):2641–2651.6. Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996. 1:23–28.7. Amin V, Cumming DV, Latchman DS. Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci Lett. 1996. 206:45–48.8. Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70). Mol Med Today. 1999. 5:525–531.9. Tezel G, Yang J, Wax MB. Heat shock proteins, immunity and glaucoma. Brain Res Bull. 2004. 62:473–480.10. Van Bergen NJ, Wood JP, Chidlow G, et al. Recharacterization of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009. 50:4267–4272.11. Mizumoto K, Rothman RJ, Farber JL. Programmed cell death (apoptosis) of mouse fibroblasts is induced by the topoisomerase II inhibitor etoposide. Mol Pharmacol. 1994. 46:890–895.12. Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988. 241:1817–1820.13. Rordorf G, Koroshetz WJ, Bonventre JV. Heat shock protects cultured neurons from glutamate toxicity. Neuron. 1991. 7:1043–1051.14. Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2001. 42:1522–1530.15. Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003. 44:1982–1992.16. Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002. 28:267–275.17. Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000. 6:435–442.18. Guzhova I, Kislyakova K, Moskaliova O, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001. 914:66–73.19. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002. 2:185–194.20. Calderwood SK, Mambula SS, Gray PJ Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007. 1113:28–39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The Effect of Heat Shock Protein 70.1 gene (hsp70.1) on Retinal Ganglion Cell Damage after Partial Lesion of the Optic Nerve in Mice