Korean J Gastroenterol.
2013 Mar;61(3):136-146. 10.4166/kjg.2013.61.3.136.
Current Status of Molecular Targeted Therapies in Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. gudwns21@medimail.co.kr
- KMID: 1501636
- DOI: http://doi.org/10.4166/kjg.2013.61.3.136
Abstract
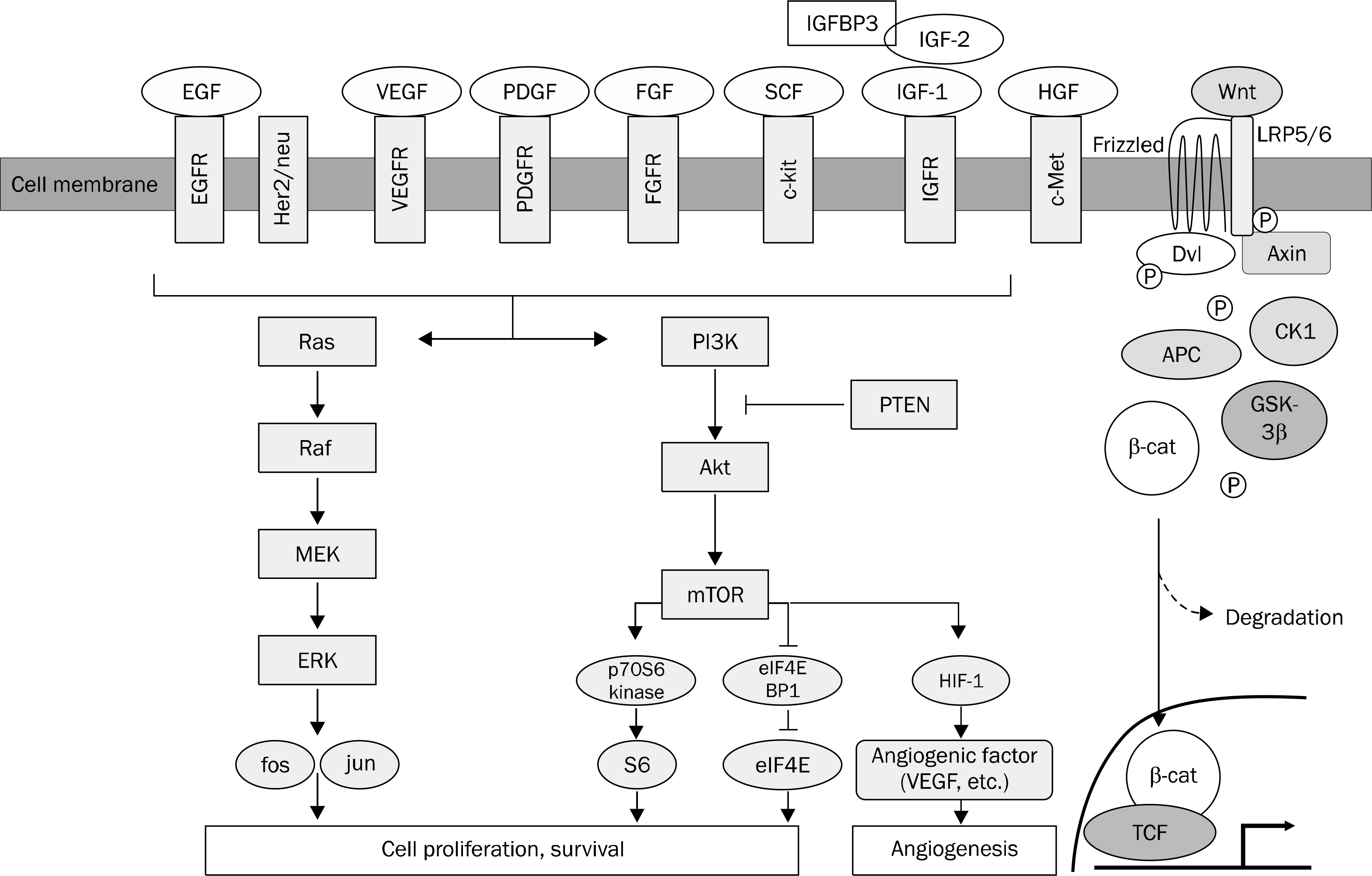
- Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death in Korea. Curative treatment is only possible when the disease is diagnosed at the early stage. The prognosis of patients with HCC is even dismal in advanced stages. No systemic cytotoxic chemotherapy has proven to be beneficial in overall survival. Recently, the understanding of the molecular pathogenesis led to the development of new therapies. With the evidence of dysregulation of critical genes associated with cellular proliferation, growth factor signaling, cell cycling, apoptosis, and angiogenesis in HCC, a number of molecular target agents are under clinical trials. Sorafenib is the first systemic anticancer drug which has proven to gain survival benefit in the global as well as Asia-Pacific trials. However, the survival gain is still modest, and further efforts to improve outcomes in patients with HCC are necessary by developing novel drugs or combining other forms of therapies. This article will review signaling pathways in HCC and introduce molecular target agents under investigation currently.
Keyword
MeSH Terms
-
Antineoplastic Agents/therapeutic use
Carcinoma, Hepatocellular/*drug therapy/metabolism/pathology
Humans
Liver Neoplasms/*drug therapy/metabolism/pathology
Mitogen-Activated Protein Kinase Kinases/antagonists & inhibitors/metabolism
Molecular Targeted Therapy
Niacinamide/analogs & derivatives/therapeutic use
Phenylurea Compounds/therapeutic use
Protein Kinase Inhibitors/therapeutic use
Proto-Oncogene Proteins c-akt/antagonists & inhibitors/metabolism
Receptor, IGF Type 1/antagonists & inhibitors/metabolism
Signal Transduction
TOR Serine-Threonine Kinases/antagonists & inhibitors/metabolism
Wnt Proteins/antagonists & inhibitors/metabolism
Antineoplastic Agents
Phenylurea Compounds
Protein Kinase Inhibitors
Wnt Proteins
Niacinamide
TOR Serine-Threonine Kinases
Receptor, IGF Type 1
Proto-Oncogene Proteins c-akt
Mitogen-Activated Protein Kinase Kinases
Figure
Reference
-
References
1. Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article2. Kudo M. Signaling pathway/molecular targets and new targeted agents under development in hepatocellular carcinoma. World J Gastroenterol. 2012; 18:6005–6017.
Article3. Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008; 48:1312–1327.
Article4. Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007; 26:3291–3310.
Article5. Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997; 236:54–58.
Article6. Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–1128.
Article7. Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Overexpression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003; 3:19.
Article8. Ito Y, Sasaki Y, Horimoto M, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998; 27:951–958.
Article9. Tsuboi Y, Ichida T, Sugitani S, et al. Overexpression of extracellular signal-regulated protein kinase and its correlation with proliferation in human hepatocellular carcinoma. Liver Int. 2004; 24:432–436.
Article10. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009; 9:550–562.
Article11. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008; 7:504–516.
Article12. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008; 358:1160–1174.
Article13. Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008; 129:245–251.
Article14. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.
Article15. Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000; 52:237–268.16. Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009; 50:604–620.
Article17. Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and metaanalysis. Br J Cancer. 2009; 100:1385–1392.
Article18. Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004; 15:215–228.
Article19. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999; 79:1283–1316.
Article20. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003; 299:708–710.21. Clarke ID, Dirks PB. A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene. 2003; 22:722–733.22. Dabrow MB, Francesco MR, McBrearty FX, Caradonna S. The effects of platelet-derived growth factor and receptor on normal and neoplastic human ovarian surface epithelium. Gynecol Oncol. 1998; 71:29–37.
Article23. Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate. 1996; 29:282–286.24. Sulzbacher I, Birner P, Träxler M, Marberger M, Haitel A. Expression of platelet-derived growth factoralpha alpha receptor is associated with tumor progression in clear cell renal cell carcinoma. Am J Clin Pathol. 2003; 120:107–112.25. Friedman SL. Closing in on the signals of hepatic fibrosis. Gastroenterology. 1997; 112:1406–1409.
Article26. Ikura Y, Morimoto H, Ogami M, Jomura H, Ikeoka N, Sakurai M. Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J Gastroenterol. 1997; 32:496–501.
Article27. Gilbertson DG, Duff ME, West JW, et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem. 2001; 276:27406–27414.28. Campbell JS, Hughes SD, Gilbertson DG, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005; 102:3389–3394.
Article29. Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptoralpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007; 6:1932–1941.30. Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997; 45:1005–1019.
Article31. Desnoyers LR, Pai R, Ferrando RE, et al. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene. 2008; 27:85–97.
Article32. French DM, Lin BC, Wang M, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One. 2012; 7:e36713.
Article33. Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003; 35:685–693.
Article34. Chen YW, Boyartchuk V, Lewis BC. Differential roles of insulin-like growth factor receptor- and insulin receptor-mediated signaling in the phenotypes of hepatocellular carcinoma cells. Neoplasia. 2009; 11:835–845.
Article35. Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001; 92:136–145.36. Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006; 66:11851–11858.
Article37. Woo HY, Heo J. Sorafenib in liver cancer. Expert Opin Pharmacother. 2012; 13:1059–1067.
Article38. Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008; 99:159–165.
Article39. Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009; 27:1800–1805.
Article40. Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006; 24:4293–4300.
Article41. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article42. Bruix J, Cheng AL, Kang YK. Effect of macroscopic vascular invasion (MVI), extrahepatic spread (EHS), and ECOG performance status (ECOG PS) on outcome in patients with advanced hepatocellular carcinoma (HCC) treated with sorafenib: analysis of two phase III, randomized, double-blind trials. J Clin Oncol. 2009; 27(15 Suppl):521s. abstr 4580.
Article43. Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012; 48:1452–1465.
Article44. Marrero J, Lencioni R, Kudo M. Global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) second interim analysis in more than 1,500 patients: clinical findings in patients with liver dysfunction. J Clin Oncol. 2011; 29(Suppl):abstr 4001.
Article45. Iavarone M, Cabibbo G, Piscaglia F, et al. SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011; 54:2055–63.
Article46. Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012; 57:101–107.
Article47. Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010; 15:85–92.
Article48. Bettinger D, Schultheiss M, Knüppel E, Thimme R, Blum HE, Spangenberg HC. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2012; 56:789–790.
Article49. Wentz SC, Wu H, Yip-Schneider MT, et al. Targeting MEK is effective chemoprevention of hepatocellular carcinoma in TGF-alpha-transgenic mice. J Gastrointest Surg. 2008; 12:30–37.50. O'Neil BH, Goff LW, Kauh JS, et al. Phase II study of the mi-togen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011; 29:2350–2356.51. Chen KF, Chen HL, Tai WT, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011; 337:155–161.
Article52. Treiber G. mTOR inhibitors for hepatocellular cancer: a for-ward-moving target. Expert Rev Anticancer Ther. 2009; 9:247–261.
Article53. Schöniger-Hekele M, Müller C. Pilot study: rapamycin in ad vanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2010; 32:763–768.54. Huynh H, Chow KH, Soo KC, et al. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009; 13:1371–1380.
Article55. Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011; 117:5094–5102.
Article56. Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008; 26:2992–2998.
Article57. Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011; 17:1973–1983.
Article58. Johnson P, Qin S, Park J, et al. Brivanib (BRI) versus sorafenib (SOR) as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma (HCC): results from the phase 3 BRISK-FL study. Hepatology. 2012; 56(Suppl):1519A.59. Llovet JM, Decaens T, Raoul JL, et al. Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study [abstract]. J Hepatol. 2012; 56(Suppl 2):S549.60. Toh HC, Chen PJ, Carr BI, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013; 119:380–387.
Article61. Hsu C, Yang TS, Huo TI, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled study. J Hepatol. 2012; 56:1097–1103.
Article62. Yau T, Chen PJ, Chan P, et al. Phase I dose-finding study of pazo-panib in hepatocellular carcinoma: evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res. 2011; 17:6914–6923.
Article63. Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009; 10:794–800.
Article64. Philip PA, Mahoney MR, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005; 23:6657–6663.
Article65. Thomas MB, Chadha R, Glover K, et al. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007; 110:1059–1067.
Article66. Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009; 64:777–783.
Article67. Bekaii-Saab T, Markowitz J, Prescott N, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009; 15:5895–5901.
Article68. Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009; 27:843–850.
Article69. Yau T, Wong H, Chan P, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs. 2012; 30:2384–2390.
Article70. Asnacios A, Fartoux L, Romano O, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer. 2008; 112:2733–2739.71. Chiorean EG, Ramasubbaiah R, Yu M, et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06–101. Oncologist. 2012; 17:13–e26.
Article72. Higano CS, Yu EY, Whiting SH, Gordon MS. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007; 25(18 Suppl):abstr 3505.
Article73. Abou-Alfa G, Gansukh B, Chou JF, et al. Phase II study of cixutumumab (IMC-A12, NSC742460;C) in hepatocellular carcinoma (HCC). J Clin Oncol. 2011; 29(Suppl):abstr 4043.74. Faivre S, Fartoux L, Bumsel F, et al. Phase I safety, and pharmacokinetic study of AVE1642, a human monoclonal antibody inhibiting the insulin-like growth factor-1 receptor (IGF-1R/CD221), administered as single agent and in combination with sorafenib as first line therapy in patients with advanced hepatocellular carcinoma (HCC) [abstract]. Hepatology. 2010; 52(Suppl):abstr 288.75. Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009; 1:1153–1171.
Article76. Richly H, Kupsch P, Passage K, et al. Results of a phase I trial of BAY 43–9006 in combination with doxorubicin in patients with primary hepatic cancer. Int J Clin Pharmacol Ther. 2004; 42:650–651.
Article77. Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010; 304:2154–2160.78. Petrini I, Lencioni M, Ricasoli M, et al. Phase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2012; 69:773–780.
Article79. Hsu CH, Shen YC, Lin ZZ, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010; 53:126–131.
Article80. Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006; 24:1898–1903.
Article81. Kaseb AO, Garrett-Mayer E, Morris JS, et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology. 2012; 82:67–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second-line treatment
- The Genomic Landscape and Its Clinical Implications in Hepatocellular Carcinoma
- Recent advances in systemic chemotherapy of hepatocellular carcinoma
- Intrahepatic cholangiocarcinoma: Tumour heterogeneity and its clinical relevance