Obstet Gynecol Sci.
2013 Nov;56(6):382-388. 10.5468/ogs.2013.56.6.382.
Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gyeongsang National University School of Medicine, Jinju, Korea. wypaik@gnu.ac.kr
- 2Institute of Health Science, Gyeongsang National Universtiy, Jinju, Korea.
- KMID: 1500372
- DOI: http://doi.org/10.5468/ogs.2013.56.6.382
Abstract
OBJECTIVE
To investigate the effect of the dimethyl sulfoxide (DMSO) and EFS-40 during vitrification on the expression of angiogenic factors in vitrified mouse ovarian tissue.
METHODS
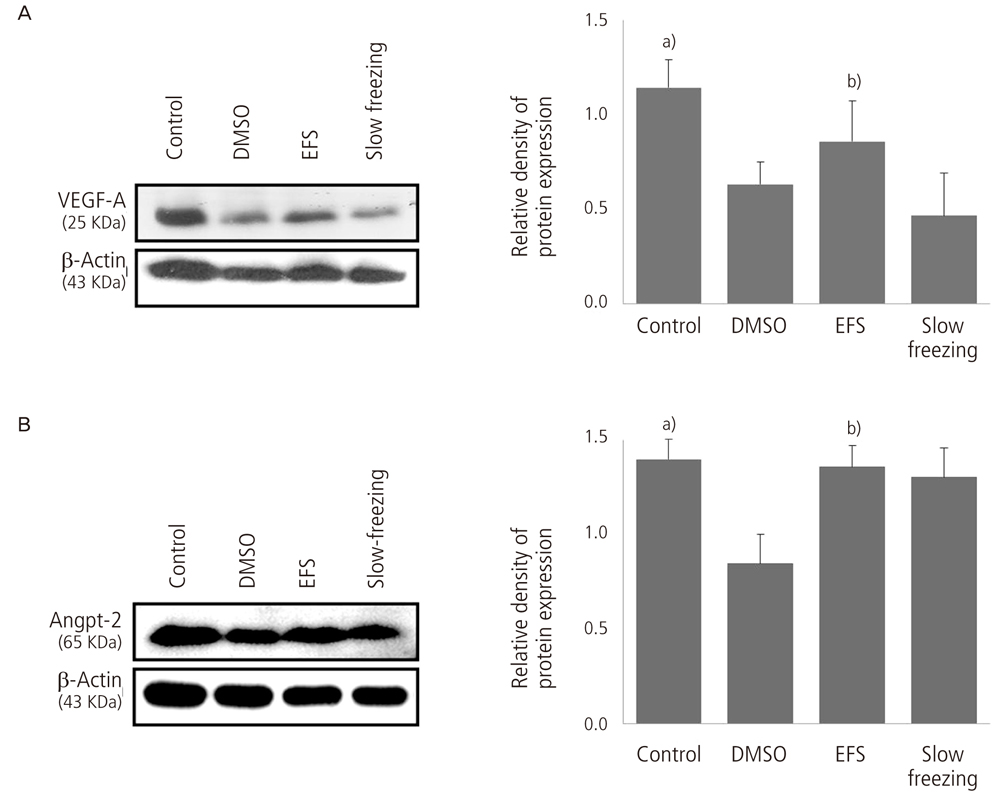
The ovarian tissues were obtained from 5 or 6 weeks aged ICR mouse. Ovarian tissues were divided into four groups: ovarian tissue without cryopreservation (control, group I), ovarian tissue vitrified with 15% DMSO (group II), ovarian tissue vitrified with EFS-40 (group III), and ovarian tissue slowly frozen with 10% DMSO (group IV). Thawing was carried out at room temperature. Levels of messenger RNA (mRNA) and protein for vascular endothelial growth factor-A (VEGF-A) and angiopoietin-2 (Angpt-2) were checked in ovarian tissues of four groups recovered on day 7 after cryopreservation. Reverse transcription-polymerase chain reaction and Western blot analysis were used to identify the levels of angiogenic factors in mouse ovarian tissues.
RESULTS
Levels of mRNA and protein for VEGF-A and Angpt-2 were significantly decreased in cryopreserved group (group II, III and IV) than control group (group I) (P< 0.05). The significant differences of levels of mRNA and protein for VEGF-A and Angpt-2 between cryopreservation methods were observed (P< 0.05). Group III showed highest expression of mRNA and protein for VEFG-A and Angpt-2 than other cryopreservation groups (P< 0.05).
CONCLUSION
These findings suggest that EFS-40 is more efficient vitrification solution for preservation of angiogenic factors than 15% DMSO during vitrification of mouse ovarian tissue. Future studies should investigate to improve the vitrification solution for ovarian tissue vitrification.
MeSH Terms
-
Angiopoietin-2
Animals
Blotting, Western
Cryopreservation
Dimethyl Sulfoxide
Female
Methods
Mice*
Mice, Inbred ICR
Ovary
Reverse Transcriptase Polymerase Chain Reaction
RNA, Messenger
Vascular Endothelial Growth Factor A
Vitrification*
Angiopoietin-2
Dimethyl Sulfoxide
RNA, Messenger
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999; 33:29–33.2. Aubard Y, Poirot C, Piver P, Galinat S, Teissier MP. Are there indications for ovarian tissue cryopreservation? Fertil Steril. 2001; 76:414–415.3. Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol. 2004; 113:Suppl 1. S33–S40.4. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001; 286:1490–1493.5. Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003; 79:594–602.6. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248.7. Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999; 5:1115–1121.8. Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999; 13:1055–1066.9. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.10. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463.11. Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999; 18:5356–5362.12. Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998; 83:233–240.13. Nishigaki A, Okada H, Tsuzuki T, Cho H, Yasuda K, Kanzaki H. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011; 96:1378–1383.14. Milenkovic M, Diaz-Garcia C, Wallin A, Brannstrom M. Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertil Steril. 2012; 97:1176–1182.15. Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod. 1995; 10:2334–2338.16. Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002; 77:403–408.17. Almodin CG, Minguetti-Camara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, et al. Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod. 2004; 19:1287–1293.18. Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003; 18:1165–1172.19. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999; 140:462–471.20. Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000; 74:122–129.21. Aubard Y, Piver P, Cogni Y, Fermeaux V, Poulin N, Driancourt MA. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999; 14:2149–2154.22. Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999; 237:1–30.23. Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012; 27:474–482.24. Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007; 55:261–268.25. Santos RR, Tharasanit T, Van Haeften T, Figueiredo JR, Silva JR, Van den Hurk R. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res. 2007; 327:167–176.26. Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009; 24:1670–1683.27. Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, et al. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010; 149:63–67.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitrification and Ultrarapid Freezing of Day 2 Mouse Embryos
- Expression of angiogenic factors in cryopreserved mouse ovaries after heterotopic autotransplantation
- Vitrification solution without sucrose for cryopreservation in mouse blastocysts
- Vitrification of Mouse Embryos in Ethylene Glycol-based Solutions
- Effect of Antifreeze Protein on Mouse Ovarian Tissue Cryopreservation and Transplantation