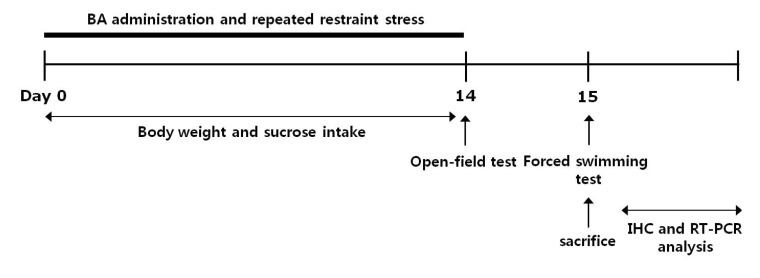
Korean J Physiol Pharmacol.
2013 Oct;17(5):393-403. 10.4196/kjpp.2013.17.5.393.
Chronic Administration of Baicalein Decreases Depression-Like Behavior Induced by Repeated Restraint Stress in Rats
- Affiliations
-
- 1Acupuncture and Meridian Science Research Center, College of Oriental Medicine, Kyung Hee University, Seoul 130-701, Korea. bombi@khu.ac.kr, dhhahm@khu.ac.kr
- 2The Graduate School of Basic Science of Oriental Medicine, College of Oriental Medicine, Kyung Hee University, Seoul 130-701, Korea.
- KMID: 1500233
- DOI: http://doi.org/10.4196/kjpp.2013.17.5.393
Abstract
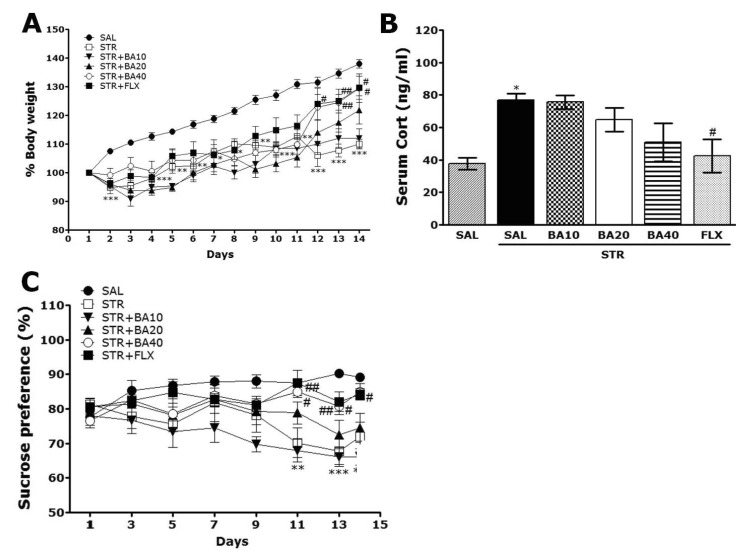
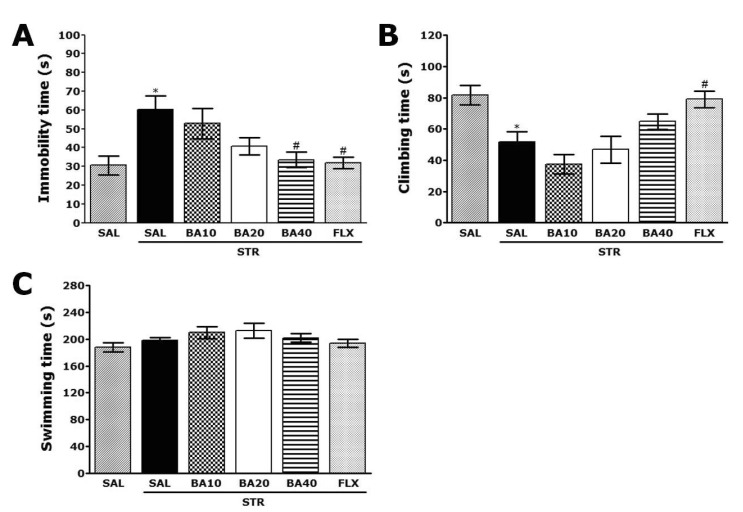
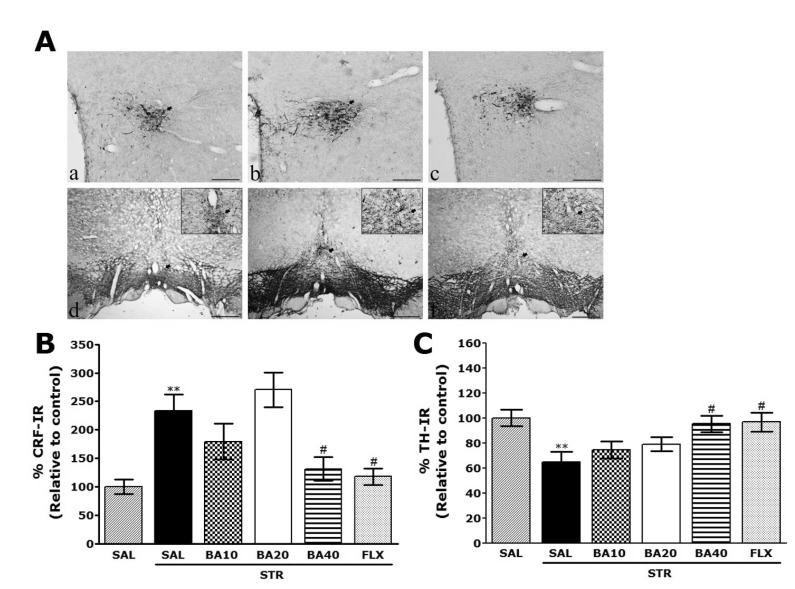
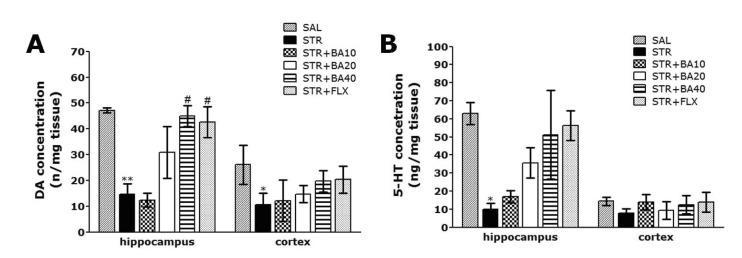
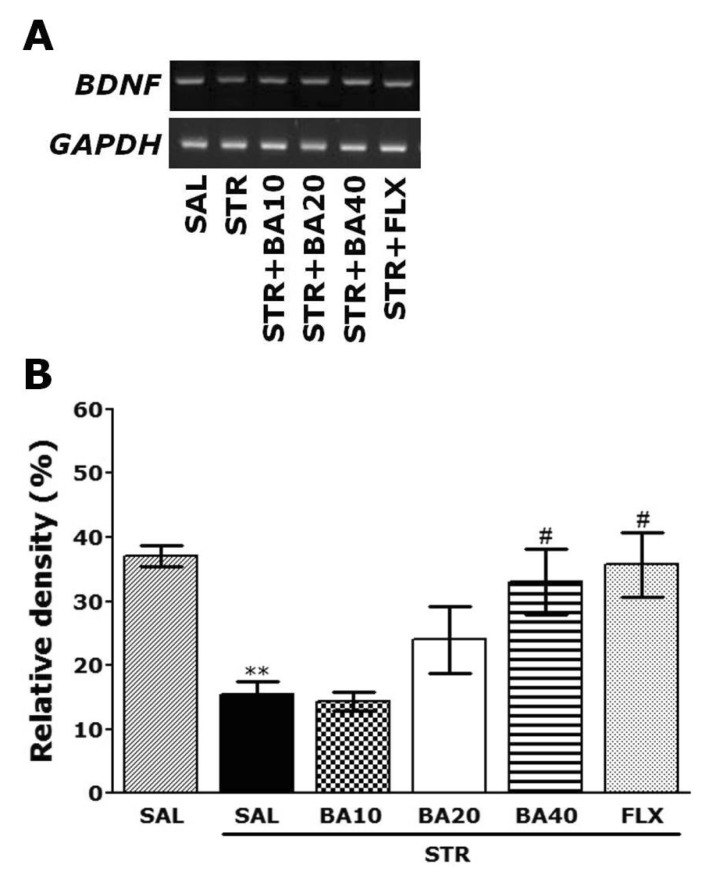
- Baicalein (BA), a plant-derived active flavonoid present in the root of Scutellaria baicalensis, has been widely used for the treatment of stress-related neuropsychiatric disorders including depression. Previous studies have demonstrated that repeated restraint stress disrupts the activity of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in depression. The behavioral and neurochemical basis of the BA effect on depression remain unclear. The present study used the forced swimming test (FST) and changes in brain neurotransmitter levels to confirm the impact of BA on repeated restraint stress-induced behavioral and neurochemical changes in rats. Male rats received 10, 20, or 40 mg/kg BA (i.p.) 30 min prior to daily exposure to repeated restraint stress (2 h/day) for 14 days. Activation of the HPA axis in response to repeated restraint stress was confirmed by measuring serum corticosterone levels and the expression of corticotrophin-releasing factor in the hypothalamus. Daily BA administration significantly decreased the duration of immobility in the FST, increased sucrose consumption, and restored the stress-related decreases in dopamine concentrations in the hippocampus to near normal levels. BA significantly inhibited the stress-induced decrease in neuronal tyrosine hydroxylase immunoreactivity in the ventral tegmental area and the expression of brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus. Taken together, these findings indicate that administration of BA prior to the repeated restraint stress significantly improves helpless behaviors and depressive symptoms, possibly by preventing the decrease in dopamine and BDNF expression. Thus, BA may be a useful agent for the treatment or alleviation of the complex symptoms associated with depression.
MeSH Terms
-
Animals
Brain
Brain-Derived Neurotrophic Factor
Corticosterone
Depression
Dopamine
Flavanones*
Hippocampus
Hypothalamus
Male
Neurons
Neurotransmitter Agents
Rats*
RNA, Messenger
Physical Exertion
Scutellaria baicalensis
Sucrose
Tyrosine 3-Monooxygenase
Ventral Tegmental Area
Brain-Derived Neurotrophic Factor
Corticosterone
Dopamine
Flavanones
Neurotransmitter Agents
RNA, Messenger
Sucrose
Tyrosine 3-Monooxygenase
Figure
Cited by 1 articles
-
Chronic Non-Social Stress Affects Depressive Behaviors But Not Anxiety in Mice
Sang Ho Yoon, Byung-Hak Kim, Sang-Kyu Ye, Myoung-Hwan Kim
Korean J Physiol Pharmacol. 2014;18(3):263-268. doi: 10.4196/kjpp.2014.18.3.263.
Reference
-
1. Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, Hu ZL, Fu H, Wang F, Chen JG. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011; 34:253–259. PMID: 21415537.
Article2. Chandrashekar N, Selvamani A, Subramanian R, Pandi A, Thiruvengadam D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol Appl Pharmacol. 2012; 261:10–21. PMID: 22369883.
Article3. Park SJ, Kim DH, Kim JM, Shin CY, Cheong JH, Ko KH, Ryu JH. Mismatch between changes in baicalein-induced memory-related biochemical parameters and behavioral consequences in mouse. Brain Res. 2010; 1355:141–150. PMID: 20691671.
Article4. Wang W, Wang F, Yang YJ, Hu ZL, Long LH, Fu H, Xie N, Chen JG. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol. 2011; 162:1364–1379. PMID: 21133890.
Article5. Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br J Pharmacol. 2002; 137:1314–1320. PMID: 12466241.
Article6. Oh SB, Park HR, Jang YJ, Choi SY, Son TG, Lee J. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br J Pharmacol. 2013; 168:421–431. PMID: 22891631.
Article7. Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on beta-amyloid peptide-(25-35)-induced amnesia in mice. Eur J Pharmacol. 2004; 506:55–61. PMID: 15588624.8. Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, Wang W, Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007; 86:423–430. PMID: 17289131.
Article9. Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010; 112:1500–1512. PMID: 20050973.
Article10. Guo QL, Ding QL, Wu ZQ. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol Pharm Bull. 2004; 27:333–337. PMID: 14993798.
Article11. Colman I, Ataullahjan A. Life course perspectives on the epidemiology of depression. Can J Psychiatry. 2010; 55:622–632. PMID: 20964941.
Article12. Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Sutton JM, Griffith JW, Rose R, Waters A, Hammen C. The role of neuroticism and extraversion in the stress-anxiety and stress-depression relationships. Anxiety Stress Coping. 2010; 23:363–381. PMID: 19890753.
Article13. Skórzewska A, Bidziński A, Lehner M, Turzyńska D, Wisłowska-Stanek A, Sobolewska A, Szyndler J, Maciejak P, Taracha E, Płaźnik A. The effects of acute and chronic administration of corticosterone on rat behavior in two models of fear responses, plasma corticosterone concentration, and c-Fos expression in the brain structures. Pharmacol Biochem Behav. 2006; 85:522–534. PMID: 17107707.14. Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008; 33:54–67. PMID: 17976922.
Article15. Huang Z, Zhong XM, Li ZY, Feng CR, Pan AJ, Mao QQ. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett. 2011; 493:145–148. PMID: 21334417.
Article16. Lee B, Shim I, Lee HJ, Yang Y, Hahm DH. Effects of acupuncture on chronic corticosterone-induced depression-like behavior and expression of neuropeptide Y in the rats. Neurosci Lett. 2009; 453:151–156. PMID: 19429024.
Article17. Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007; 1148:226–233. PMID: 17368432.
Article18. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002; 22:6810–6818. PMID: 12151561.
Article19. Mizoguchi K, Yuzurihara M, Ishige A, Aburada M, Tabira T. Saiko-ka-ryukotsu-borei-to, a herbal medicine, ameliorates chronic stress-induced depressive state in rotarod performance. Pharmacol Biochem Behav. 2003; 75:419–425. PMID: 12873634.
Article20. Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002; 27:519–525. PMID: 12199158.21. Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005; 29:525–546. PMID: 15925696.
Article22. Bravo JA, Díaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, Arancibia S, Fiedler JL. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol. 2009; 20:273–285. PMID: 19424057.
Article23. O'Mahony CM, Clarke G, Gibney S, Dinan TG, Cryan JF. Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression. Pharmacol Biochem Behav. 2011; 97:690–699. PMID: 21110995.24. Zhu KY, Mao QQ, Ip SP, Choi RC, Dong TT, Lau DT, Tsim KW. A standardized chinese herbal decoction, kai-xin-san, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med. 2012; 2012:149256. PMID: 22973399.
Article25. Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005; 82:200–206. PMID: 16171853.
Article26. Molina-Hernández M, Téllez-Alcántara NP, Olivera-López JI, Jaramillo MT. Intra-lateral septal infusions of folic acid alone or combined with various antidepressant drugs produce antidepressant-like actions in male Wistar rats forced to swim. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 36:78–84. PMID: 21907753.
Article27. Lee B, Shim I, Lee H, Hahm DH. Effect of ginsenoside Re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J Microbiol Biotechnol. 2012; 22:708–720. PMID: 22561867.
Article28. Mao QQ, Ip SP, Ko KM, Tsai SH, Che CT. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33:1211–1216. PMID: 19596036.
Article29. Gmeiner BM, Seelos CC. Measurement of phosphotyrosine phosphatase activity using the Folin-Ciocalteu phenol reaction. Biochem Mol Biol Int. 1995; 36:943–948. PMID: 7581010.30. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3th ed. New York, USA: Academic Press;1986. p. 54–85.31. Ling S, Jamali F. Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci. 2003; 6:246–251. PMID: 12935437.32. Sun H, Che QM, Zhao X, Pu XP. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur J Pharmacol. 2010; 631:53–60. PMID: 20079350.
Article33. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39:112–119. PMID: 22664354.
Article34. Nacher J, Pham K, Gil-Fernandez V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004; 126:503–509. PMID: 15207367.
Article35. Sigwalt AR, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, Cadore EL, Lhullier FL, de Bem AF, Hohl A, de Matos FJ, de Oliveira PA, Prediger RD, Guglielmo LG, Latini A. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011; 192:661–674. PMID: 21712072.
Article36. Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006; 17:271–287. PMID: 16572005.
Article37. Ulloa JL, Castañeda P, Berríos C, Díaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010; 97:213–221. PMID: 20705085.
Article38. Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012; 226:459–474. PMID: 22986163.
Article39. Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G946–G953. PMID: 20689056.
Article40. Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004; 82:751–762. PMID: 15327926.
Article41. Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007; 146:1734–1742. PMID: 17481824.
Article42. Park HJ, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I. Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol. 2010; 14:371–376. PMID: 21311677.43. Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012; 217:337–351. PMID: 22002740.
Article44. Sevgi S, Ozek M, Eroglu L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find Exp Clin Pharmacol. 2006; 28:95–99. PMID: 16636719.45. Spasojevic N, Gavrilovic L, Dronjak S. Effects of repeated maprotiline and fluoxetine treatment on gene expression of catecholamine synthesizing enzymes in adrenal medulla of unstressed and stressed rats. Auton Autacoid Pharmacol. 2010; 30:213–217. PMID: 20626387.
Article46. Shishkina GT, Kalinina TS, Dygalo NN. Effects of swim stress and fluoxetine on 5-HT1A receptor gene expression and monoamine metabolism in the rat brain regions. Cell Mol Neurobiol. 2012; 32:787–794. PMID: 22453856.
Article47. Cui J, Jiang L, Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012; 26:697–713. PMID: 21948936.
Article48. Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004; 1018:141–150. PMID: 15240363.
Article49. Bergström A, Jayatissa MN, Mørk A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008; 1196:41–52. PMID: 18234161.
Article50. Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, Branchi I, Alleva E. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neurosci Biobehav Rev. 2010; 34:808–820. PMID: 20171244.
Article51. Dagnino-Subiabre A, Zepeda-Carreño R, Díaz-Véliz G, Mora S, Aboitiz F. Chronic stress induces upregulation of brain-derived neurotrophic factor (BDNF) mRNA and integrin alpha5 expression in the rat pineal gland. Brain Res. 2006; 1086:27–34. PMID: 16626638.52. Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009; 14:681–695. PMID: 19153574.
Article53. Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006; 31:38–48. PMID: 15996825.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repeated Short-term (2hx14d) Emotional Stress Induces Lasting Depression-like Behavior in Mice
- Chronic Restraint Stress Decreases the Excitability of Hypothalamic POMC Neuron and Increases Food Intake
- Chronic Administration of Bacopa Monniera Increases BDNF Protein and mRNA Expressions: A Study in Chronic Unpredictable Stress Induced Animal Model of Depression
- Reversion of BDNF, Akt and CREB in Hippocampus of Chronic Unpredictable Stress Induced Rats: Effects of Phytochemical, Bacopa Monnieri
- The Effects of Astragalus Membranaceus on Repeated Restraint Stress-induced Biochemical and Behavioral Responses