Korean J Physiol Pharmacol.
2012 Oct;16(5):293-296. 10.4196/kjpp.2012.16.5.293.
Effect of Intensity of Unconditional Stimulus on Reconsolidation of Contextual Fear Memory
- Affiliations
-
- 1National Creative Research Initiative Center for Memory, Department of Biological Sciences, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea. kaang@snu.ac.kr
- 2Department of Anatomy, Graduate School of Medicine, Brain Science & Engineering Institute, Kyungpook National University, Daegu 700-842, Korea.
- 3Department of Brain and Cognitive Sciences, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea.
- KMID: 1493960
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.293
Abstract
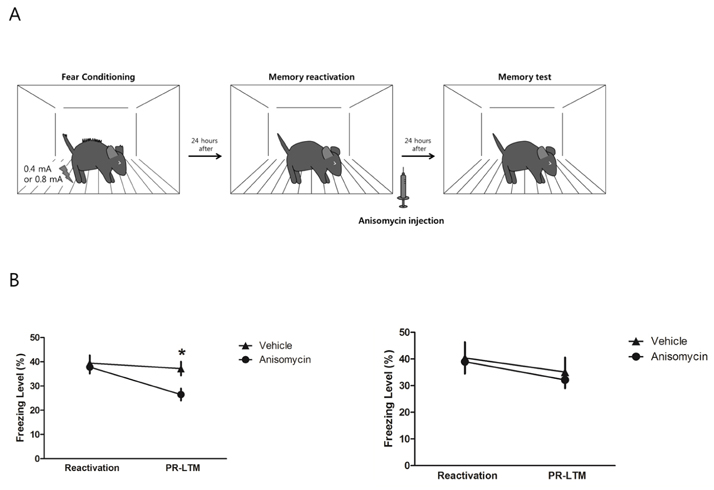
- Memory reconsolidation is ubiquitous across species and various memory tasks. It is a dynamic process in which memory is modified and/or updated. In experimental conditions, memory reconsolidation is usually characterized by the fact that the consolidated memory is disrupted by a combination of memory reactivation and inhibition of protein synthesis. However, under some experimental conditions, the reactivated memory is not disrupted by inhibition of protein synthesis. This so called "boundary condition" of reconsolidation may be related to memory strength. In Pavlovian fear conditioning, the intensity of unconditional stimulus (US) determines the strength of the fear memory. In this study, we examined the effect of the intensity of US on the reconsolidation of contextual fear memory. Strong contextual fear memory, which is conditioned with strong US, is not disrupted by inhibition of protein synthesis after its reactivation; however, a weak fear memory is often disrupted. This suggests that a US of strong intensity can inhibit reconsolidation of contextual fear memory.
Keyword
MeSH Terms
Figure
Reference
-
1. Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009. 10:224–234.2. Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000. 406:722–726.3. Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007. 8:262–275.4. Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004. 24:4787–4795.5. Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009. 12:905–912.6. Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. Retrieval does not induce reconsolidation of inhibitory avoidance memory. Learn Mem. 2004. 11:572–578.7. Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behav Neurosci. 2004. 118:956–964.8. Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci USA. 2004. 101:4667–4672.9. Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007. 10:414–416.10. Sim SE, Park SW, Choi SL, Yu NK, Ko HG, Jang DJ, Lee K, Kaang BK. Assessment of the effects of virus-mediated limited Oct4 overexpression on the structure of the hippocampus and behavior in mice. BMB Rep. 2011. 44:793–798.11. Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiol Behav. 1973. 10:555–562.12. Choi JH, Kim JE, Kaang BK. Protein synthesis and degradation are required for the incorporation of modified information into the pre-existing object-location memory. Mol Brain. 2010. 3:1.13. Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006. 9:1237–1239.14. Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008. 319:1253–1256.15. Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009. 29:402–413.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural Circuit and Mechanism of Fear Conditioning
- Differential Encoding of Trace and Delay Fear Memory in the Entorhinal Cortex
- The Three Musketeers in the Medial Prefrontal Cortex: Subregion-specific Structural and Functional Plasticity Underlying Fear Memory Stages
- Valproate Adjuvant Cognitive Behavioral Therapy in Panic Disorder Patients With Comorbid Bipolar Disorder: Case Series and Review of the Literature
- Effect of Dominant Eye and Contextual Background on Binocular Rivalry