Korean J Physiol Pharmacol.
2008 Oct;12(5):237-243. 10.4196/kjpp.2008.12.5.237.
Spinal Metabotropic Glutamate Receptors (mGluRs) are Involved in the Melittin-induced Nociception in Rats
- Affiliations
-
- 1Department of Orthopedic Surgery, School of Medicine, Keimyung University, Daegu, Korea.
- 2Department of Physiology, College of Medicine, Hanyang University, Seoul, Korea. shinhg@hanyang.ac.kr
- KMID: 1486105
- DOI: http://doi.org/10.4196/kjpp.2008.12.5.237
Abstract
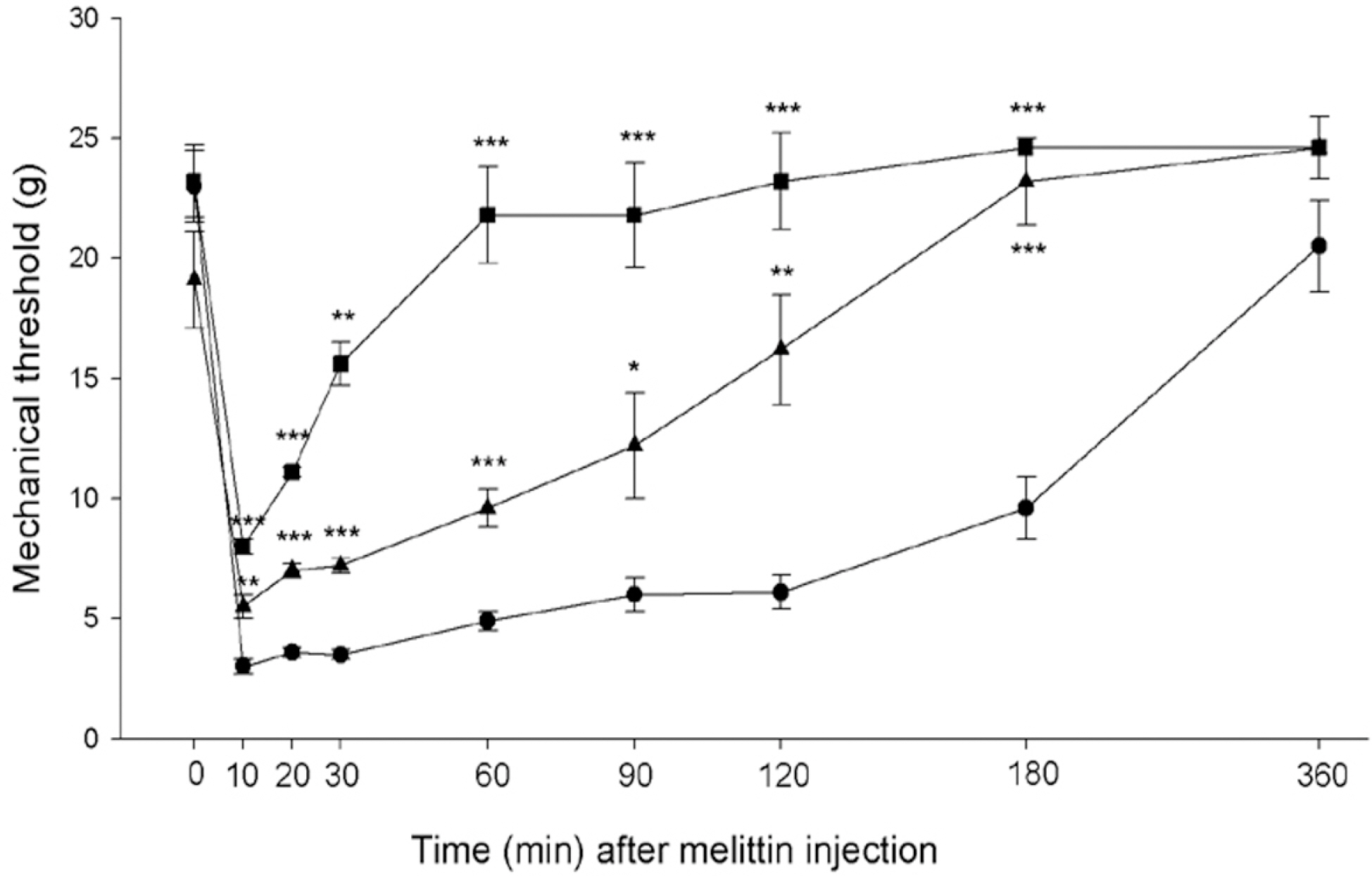
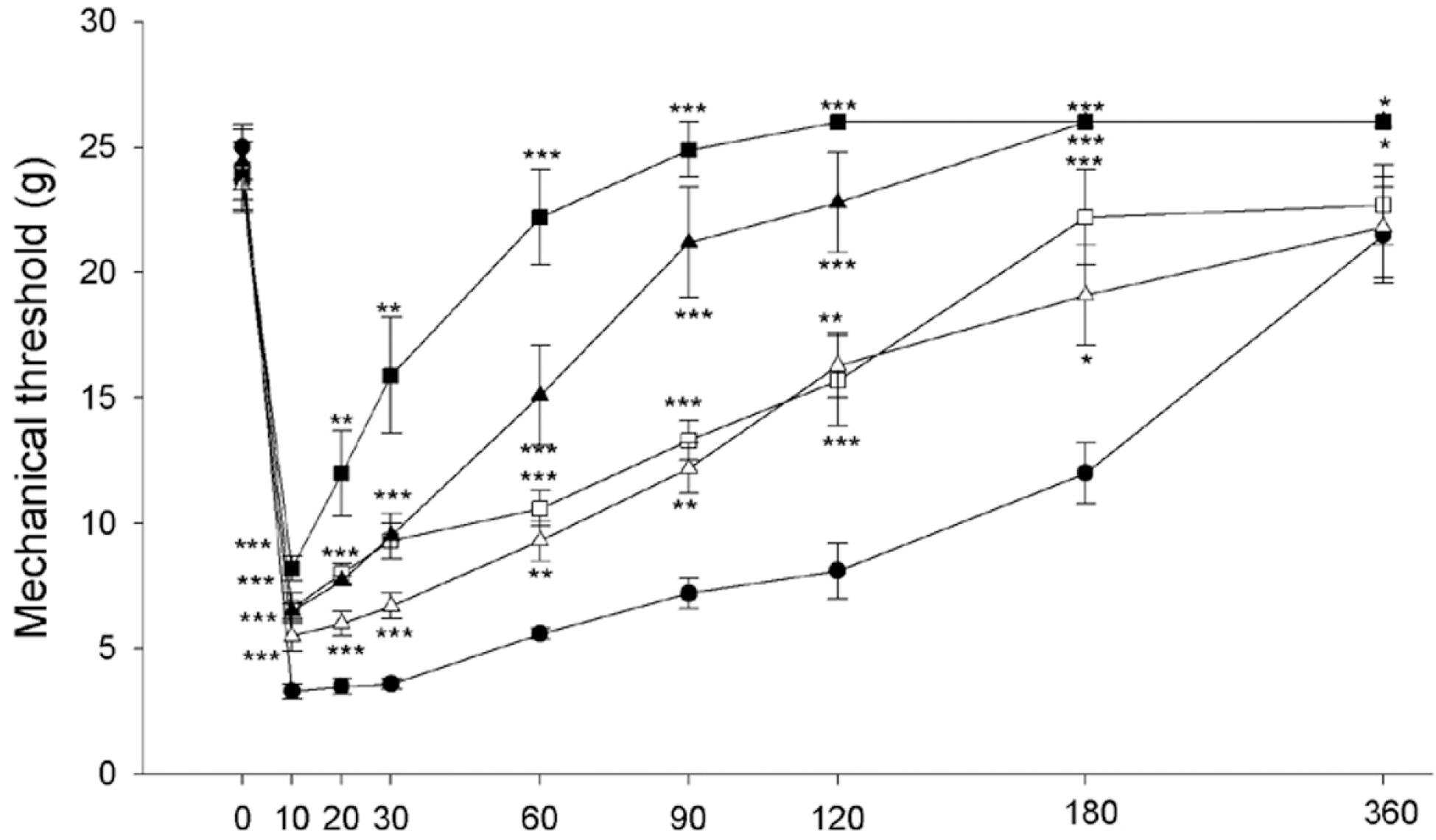
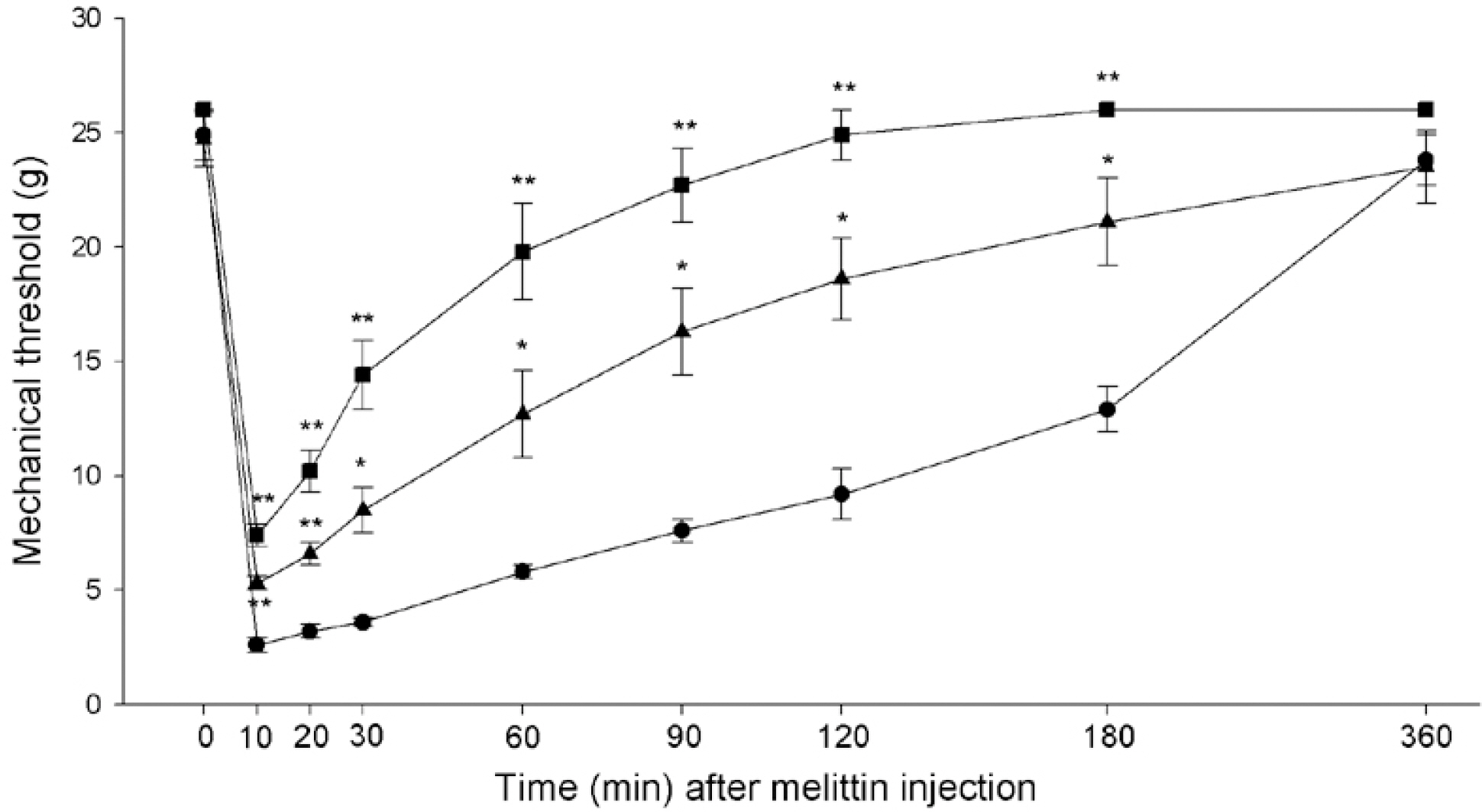
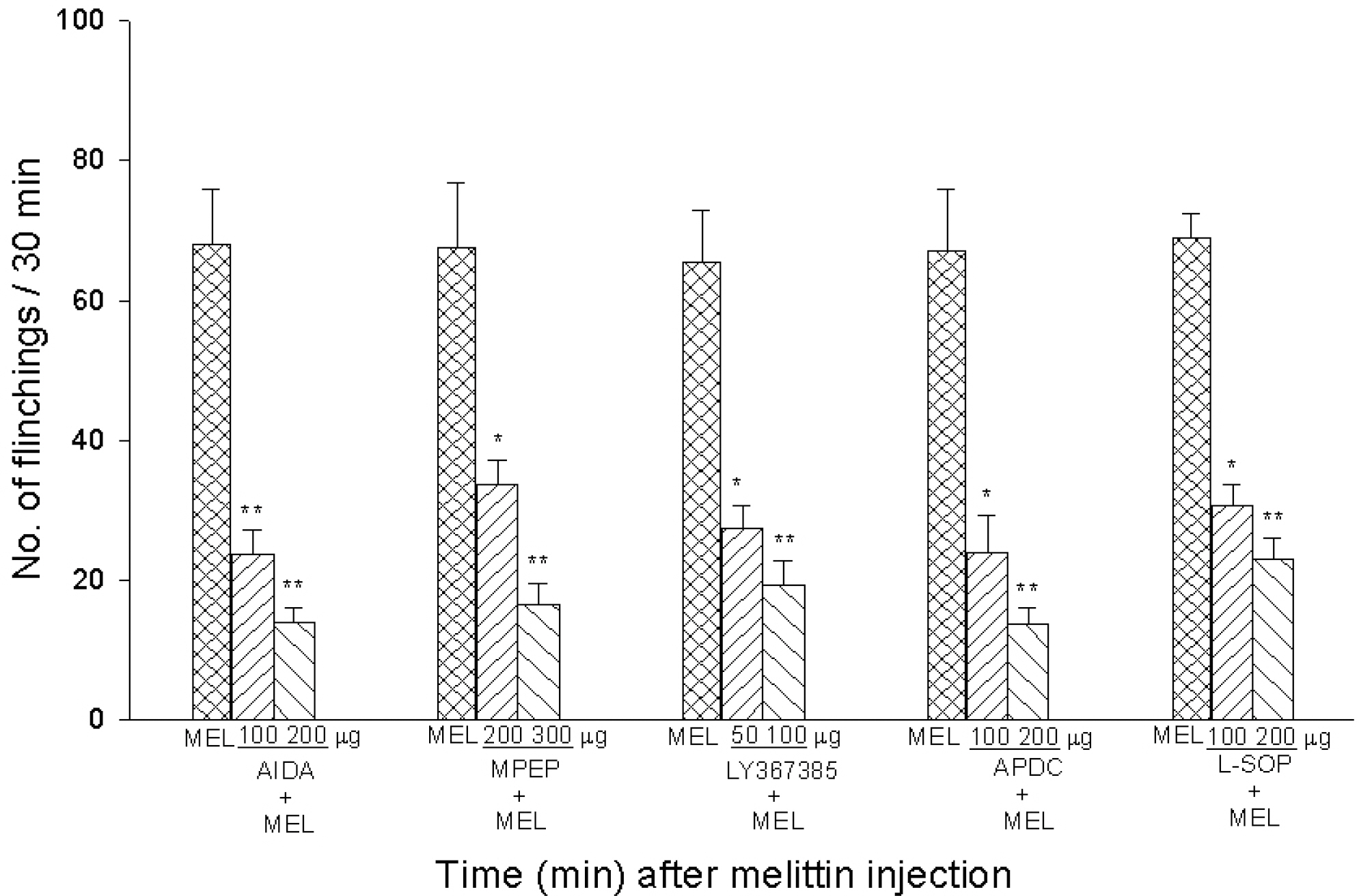
- Intraplantar injection of melittin has been known to induce sustained decrease of mechanical threshold and increase of spontaneous flinchings. The present study was undertaken to investigate how the melittin-induced nociceptive responses were modulated by changes of metabotropic glutamate receptor (mGluR) activity. Changes in paw withdrawal threshold (PWT), number of flinchings and paw thickness were measured at a given time point after injection of melittin (10microgram/paw) into the mid-plantar area of rat hindpaw. To observe the effects of mGluRs on the melittin-induced nociceptions, group I mGluR (AIDA, 100microgram and 200microgram), mGluR1 (LY367385, 50microgram and 100microgram) and mGluR5 (MPEP, 200microgram and 300microgram) antagonists, group II (APDC, 100microgram and 200microgram) and III (L-SOP, 100microgram and 200microgram) agonists were intrathecally administered 20 min before melittin injection. Intraplantar injection of melittin induced a sustained decrease of mechanical threshold, spontaneous flinchings and edema. The effects of melittin to reduce mechanical threshold and to induce spontaneous flinchings were significantly suppressed following intrathecal pre-administration of group I mGluR, mGluR1 and mGluR5 antagonists, group II and III mGluR agonists. Group I mGluR antagonists and group II and III mGluR agonists had no significant effect on melittin-induced edema. These experimental findings indicate that multiple spinal mGluRs are involved in the modulation of melittin-induced nociceptive responses.
MeSH Terms
Figure
Cited by 1 articles
-
Regulation of DREAM Expression by Group I mGluR
Jinu Lee, Insook Kim, So Ra Oh, Suk Jin Ko, Mi Kyung Lim, Dong Goo Kim, Chul Hoon Kim
Korean J Physiol Pharmacol. 2011;15(2):95-100. doi: 10.4196/kjpp.2011.15.2.95.
Reference
-
Alvarez FJ., Villalba RM., Carr PA., Grandes P., Somohano PM. Differential distribution of metabotropic glutamate receptor 1a, 1b and 5 in the rat spinal cord. J Comp Neurol. 422:464–487. 2000.Azkue JJ., Mateos JM., Elezgarai I., Benitez R., Osorio A., Diez J., Bilbao A., Bidaurrazaga A., Grandes P. The metabotropic glutamate receptor subtype mGluR2/3 is located at extrasynaptic loci in rat spinal dorsal horn synapses. Neurosci Lett. 287:236–238. 2000.Berthele A., Boxall SJ., Urban A., Anneser JHH., Zieglgansberger W., Urban L., Tolle TR. Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Develop Brain Res. 112:39–53. 1999.
ArticleBhave G., Karim F., Carlton SM., Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nature Neurosci. 4:417–423. 2001.
ArticleBoxall SJ., Berthele A., Laurie DJ., Sommer B., Zieglgansberger W., Urban L., Tolle TR. Enhanced expression of metabotropic glutmate 3 messenger RNA in the rat spinal cord during ultraviolet irradiation induced peripheral inflammation. Neuroscience. 82:591–602. 1998.Budai D., Larson AA. The involvement of metabotropic glutamate receptors in sensory transmission in dorsal horn of the rat spinal cord. Neuroscience. 83:571–580. 1998.
ArticleCarlton SM., Hargett GL., Coggeshall RE. Localization of meta-botropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 105:957–969. 2001.
ArticleChaplan SR., Bach FW., Pogrel JW., Chung JM., Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 53:55–63. 1994.
ArticleChen CY., Ling EH., Horowitz JM., Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by group II and III but not group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 538:773–786. 2002.
ArticleChen SR., Pan HL. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neuron in normal and nerve-injured rats. J Pharmacol Exp Ther. 312:120–126. 2005.Dolan S., Kelly JG., Monteiro AM., Nolan AM. Differential expression of central metabotropic glutamate receptor (mGluR) subtypes in a clinical model of post-surgical pain. Pain. 110:369–377. 2004.
ArticleDolan S., Kelly JG., Monteiro AM., Nolan AM. Upregulation of meta-botropic glutamate receptor subtypes 3 and 5 in spinal cord in a clinical model of persistent inflammation and hyperalgesia. Pain. 106:501–512. 2003.
ArticleDolan S., Nolan AM. Behavioral evidence supporting a differential role for group I and II metabotropic glutamate receptors in spinal nociceptive transmission. Neuropharmacology. 39:1132–1138. 2000.Dollan S., Nolan AM. Behavioral evidence supporting a differential role for spinal group I and II metabotropic glutamate receptors in inflammatory hyperalgesia in sheep. Neuropharmacology. 43:319–326. 2002.
ArticleEndoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 1024:212–224. 2004.
ArticleFerraguti F., Baldani-Guerra B., Corsi M., Nakanishi S., Corti C. Activation of the extracellular signal-regulated kinase 2 by meta-botropic glutamate receptors. Eur J Neurosci. 11:2073–2082. 1999.
ArticleFisher K., Lefebvre C., Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 73:411–418. 2002.
ArticleFundytus ME., Fisher K., Dray A., Henry JL., Coderre TJ. In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. Neuroreport. 9:731–735. 1998.
ArticleFundytus ME., Yashpal K., Chabot JG., Osborne MG., Lefebvre CD., Dray A., Henry JL., Coderre TJ. Knockdown of spinal meta-botropic glutamate receptor 1 (mGluR1) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 132:354–367. 2001.
ArticleGerber G., Zhong J., Youn DH., Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 100:393–406. 2000.
ArticleHeinke B., Sandkuhler J. Group I metabotropic glutamate receptor-induced Ca2+-gradients in rat superficial spinal dorsal horn neurons. Neuropharmacology. 52:1015–1023. 2007.Hu HJ., Gereau RW. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 90:1680–1688. 2003.
ArticleHua XY., Chen P., Yaksh TL. Inhibition of spinal protein kinase C reduces nerve injury-induced tactile allodynia in neuropathic rats. Neurosci Lett. 276:99–102. 1999.
ArticleHudson LJ., Bevan S., McNair K., Gentry C., Fox A., Kuhn R., Winter J. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 22:2660–2668. 2002.
ArticleJi RR., Rupp F. Phosphorylation of transcription factor CREB in the rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 17:1776–1785. 1997.Jia H., Rustioni A., Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 410:627–642. 1999.
ArticleJung CY., Choi HS., Ju JS., Park HS., Kwon TG., Bae YC., Ahn DK. Central metabotropic glutamate receptors differentially participate in interleukin-1β-induced mechanical allodynia in the orofacial area of conscious rats. J Pain. 7:747–756. 2006.
ArticleKim JH., Shin HK. N-methyl D-aspartate (NMDA) and non-NMDA receptors are involved in the production and maintenance of nociceptive responses by intraplantar injection of bee venom and melittin in the rat. Kor J Physiol Pharmacol. 9:179–186. 2005.Kim J., Shin HK., Lee KH. Melittin-induced nociceptive responses are alleviated by cyclooxygenase-1 inhibitor. Kor J Physiol Pharmacol. 10:45–50. 2006.Lariviere WR., Melzack R. The bee venom test: a new tonic-pain test. Pain. 66:271–277. 1996.
ArticleLefebvre C., Fisher K., Cahill CM., Coderre TJ. Evidence that DHPG-induced nociception depends on glutamate release from primary afferent C-fibers. Neuroreport. 11:1631–1635. 2000.Li H., Ohishi H., Kinoshita A., Shigemoto R., Nomura S., Mizuno N. Localization of a metabotropic glutamate receptor, mGluR7, in axon terminals of presumed nociceptive primary afferent fibers in the superficial layers of the spinal dorsal horn: an electron microscope study in the rat. Neurosci Lett. 223:153–156. 1997.
ArticleLi KC., Chen J. Altered pain-related behaviors and spinal neuronal responses produced by s.c. injection of melittin in rats. Neuroscience. 126:753–762. 2004.
ArticleLiang YC., Huang CC., Hsu KS. Characterization of long-term potentiation of primary afferent transmission at trigeminal synapses of juvenile rats: essential role of subtype 5 metabotropic glutamate receptors. Pain. 114:417–428. 2005.
ArticleMills CD., Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 319:59–62. 2002.
ArticleMills CD., Xu GY., McAdoo DJ., Hulsebosch CE. Involvement of metabotropic glutamate receptors in excitatory amino acid and GABA release following spinal cord injury. J Neurochem. 79:835–848. 2001.Miyabe T., Miletic V. Multiple kinase pathways mediate the early sciatic ligation-associated activation of CREB in the rat spinal dorsal horn. Neurosci Lett. 381:80–85. 2005.
ArticleNeugebauer V., Chen PS., Willis WD. Group II and III metbotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophysiol. 84:2998–3009. 2000.Neugebauer V., Lucke T., Schaible HG. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur J Neurosci. 6:1179–1186. 1994.
ArticleNoda K., Anzai T., Ogata M., Akita H., Ogura T., Saji M. Antisense knockdown of spinal mGluR1 reduces the sustained phase of formalin-induced nociceptive responses. Brain Res. 987:194–200. 2003.Ohishi H., Nomura S., Ding YQ., Shigemoto R., Wada E., Kinoshita A., Li JL., Neki A., Nakanishi S., Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunocytochemical study in the rat. Neurosci Lett. 202:85–88. 1995.Shin HK., Kim JH. Melittin selectively activates capsaicin-sensitive primary afferent fibers. Neuroreport. 15:1745–1749. 2004.
ArticleShin HK., Lee KH., Cho CH. Calcium ions are involved in modulation of melittin-induced nociception in rat: II. Effect of calcium chelator. Kor J Physiol Pharmacol. 10:297–302. 2006.Shin HK., Lee KH., Lee SE. Comperative study on the nociceptive responses induced by whole bee venom and melittin. Kor J Physiol Pharmacol. 8:281–288. 2004.Shin HK., Lee KH. Calcium ions are involved in modulation of melittin-induced nociception in rat: I. Effect of voltage-gated calcium channel antagonist. Kor J Physiol Pharmacol. 10:255–261. 2006.Shin HK., Lee SE. Multiple 5-hydroxytryptamine (5-HT) receptors are involved in the melittin-induced nociceptive responses in rat: I. Role of peripheral 5-HT receptor. Kor J Physiol Pharmacol. 11:221–226. 2007.Sluka KA., Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain. 71:165–178. 1997.
ArticleSluka KA. Activation of the cAMP transduction cascade contributes to the mechanical hyperalgesia and allodynia induced by intradermal injection of capsaicin. Br J Pharmacol. 122:1165–1173. 1997.
ArticleSoliman AC., Yu JSC., Coderre TJ. mGluR and NMDA receptor contributes to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 48:325–332. 2005.Song XJ., Wang ZB., Gan Q., Walters ET. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglion compression. J Neurophysiol. 95:479–492. 2006.Sumikura H., Andersen OK., Drewes AM., Arendt-Nielsen L. A comparison of hyperalgesia and neurogenic inflammation induced by melittin and capsaicin in humans. Neurosci Lett. 337:147–150. 2003.
ArticleTao YX., Li YQ., Zhao ZQ., Johns RA. Synaptic relationship of the neurons containing a metabotropic glutamate receptor, mGluR5, with nociceptive primary afferent and GABAergic terminals in rat spinal superficial laminae. Brain Res. 875:138–143. 2000.
ArticleYashpal K., Fisher K., Chabot JG., Coderre TJ. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 94:17–29. 2001.
ArticleYoung MR., Blackburn-Munro G., Dickinson T., Johnson MJ., Anderson H., Nakalembe I., Fleetwood-Walker SM. Antisense ablation of type I metabotropic glutamate receptor mGluR1 inhibits spinal nociceptive transmission. J Neurosci. 18:10180–10188. 1998.Young MR., Fleetwood-Walker SM., Mitchell R., Dickinson T. The involvement of metabotropic glutamate receptors and their intracellular signaling pathways in sustained nociceptive transmission in rat dorsal horn neurons. Neuropharmacology. 34:1033–1041. 1995.Yu YQ., Chen J. Activation of spinal extracellular signaling-regulated kinases by intraplantar melittin injection. Neurosci Lett. 381:194–198. 2005.
ArticleZhong J., Gerber G., Kojić L., Randić M. Dual modulation of excitatory synaptic transmission by agonists at group I metabotropic glutamate receptors in the rat spinal dorsal horn. Brain Res. 887:359–377. 2000.
ArticleZhou S., Komak S., Du J., Carlton SM. Metabotropic glutamate 1α receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 913:18–26. 2001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Intrathecal Drugs Acting on Metabotropic Glutamate Receptors and Interaction with Morphine on a Rat Incisional Pain
- Antinociceptive Effects of Intrathecal Metabotropic Glutamate Receptor Compounds and Morphine in Rats
- Potentiation of Morphine's Antinociception by Group II and Group III Metabotropic Glutamate Receptors Agonists on a Rat Incisional Pain
- Modulation of Amygdala Synaptic Transmission by Metabotropic Glutamate Receptors
- Calcium Ions are Involved in Modulation of Melittin-induced Nociception in Rat: II. Effect of Calcium Chelator