Korean J Physiol Pharmacol.
2008 Oct;12(5):225-230. 10.4196/kjpp.2008.12.5.225.
Netrin-1 Specifically Enhances Cell Spreading on Fibronectin in Human Glioblastoma Cells
- Affiliations
-
- 1Department of Physiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan, Korea. phwantae@dau.ac.kr
- 2Department of Microbiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan, Korea.
- KMID: 1486103
- DOI: http://doi.org/10.4196/kjpp.2008.12.5.225
Abstract
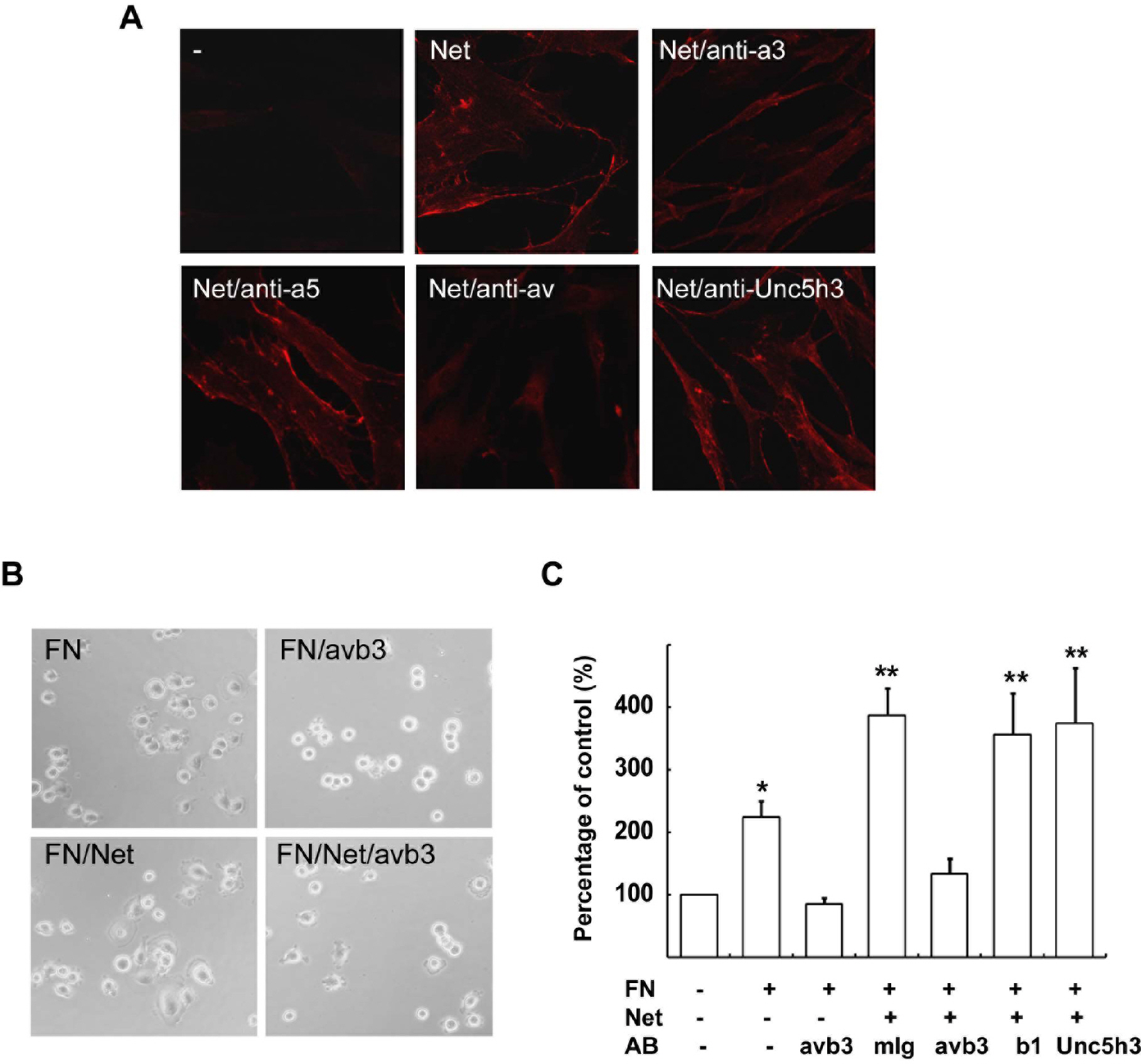
- Netrins are secreted molecules and involved in axon guidance, cell migration and tumor development. Recent studies revealed that netrins perform novel functions in such processes as epithelial development and angiogenesis without operating through the classical netrin receptors, DCC (Deleted in Colorectal Cancer) and Unc5h. In the present study, we investigated the roles of netrin-1 and its receptors in cell spreading of human glioblastoma cells, and found that netrin-1 haptotactically enhanced fibronectin-induced cell spreading and focal adhesion formation in U373 glioblastoma cells. Netrin-1 binding to the U373 cell membrane was blocked by an antibody against alpha v integrin subunit, but not by an anti-DCC or anti-Unc5h antibody. In addition, enhancement of the fibronectin response by netrin-1 was abrogated by a function blocking antibody against integrin alpha v beta 3. Since the alpha v subunit of the integrin family plays an important role in the pathophysiological aspects of cell migration, including tumor angiogenesis and metastasis, our data provide important insight into the molecular mechanism of netrin function.
Keyword
MeSH Terms
-
Axons
Cell Membrane
Cell Movement
Fibronectins
Focal Adhesions
Glioblastoma
Humans
Integrin alphaV
Integrin alphaVbeta3
Neoplasm Metastasis
Nerve Growth Factors
Receptors, Cell Surface
Tumor Suppressor Proteins
Fibronectins
Integrin alphaV
Integrin alphaVbeta3
Nerve Growth Factors
Receptors, Cell Surface
Tumor Suppressor Proteins
Figure
Reference
-
Barry ST., Critchley DR. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-delta to focal adhesions. J Cell Sci. 107:2033–2045. 1994.
ArticleCirulli V., Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Bio. 8:296–306. 2007.
ArticleColamarino SA., Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 81:621–629. 1995.
ArticleDanen EH., Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 189:1–13. 2001.
ArticleEliceiri BP., Cheresh DA. Role of alpha v integrins during angiogenesis. Cancer J. 3:S245–249. 2000.Hong K., Hinck L., Nishiyama M., Poo MM., Tessier-Lavigne M., Stein E. A ligand gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 97:927–941. 1999.Keino-Masu K., Masu M., Hinck L., Leonardo ED., Chan SS., Culotti JG., Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 87:175–185. 1996.
ArticleKennedy TE., Serafini T., dela Torre Jr., Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 78:425–435. 1994.
ArticleKim TH., Lee HK., Seo IA., Bae HR., Suh DJ., Wu J., Rao Y., Hwang KG., Park HT. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J Neurochem. 95:1–8. 2005.
ArticleLee HK., Seo IA., Park HK., Park HT. Identification of the basement membrane protein nidogen as a candidate ligand for tumor endothelial marker 7 in vitro and in vivo. FEBS Lett. 580:2253–2257. 2006.
ArticleLeonardo ED., Hinck L., Masu M., Keino-Masu K., Ackerman SL., Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 386:833–838. 1997.
ArticleLiu G., Beggs H., Jürgensen C., Park HT., Tang H., Gorski J., Jones KR., Reichardt LF., Wu J., Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 7:1222–1232. 2004.
ArticleLy NP., Komatsuzaki K., Fraser IP., Tseng AA., Prodhan P., Moore KJ., Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 102:14729–14734. 2005.
ArticlePark HT., Wu J., Rao Y. Molecular control of neuronal migration. Bioessays. 24:821–827. 2002.
ArticlePark KW., Crouse D., Lee M., Karnik SK., Sorensen LK., Murphy KJ., Kuo CJ., Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 101:16210–16215. 2004.
ArticleRodrigues S., De Wever O., Bruyneel E., Rooney RJ., Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 26:5615–5625. 2007.
ArticleSchneiders FI., Maertens B., Böse K., Li Y., Brunken WJ., Paulsson M., Smyth N., Koch M. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 282:23750–23758. 2007.
ArticleSerafini T., Kennedy TE., Galko MJ., Mirzayan C., Jessell TM., Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 78:409–424. 1994.
ArticleSerini G., Valdembri D., Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 312:651–658. 2006.
ArticleWheeler AP., Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res. 313:3505–3516. 2007.
ArticleWilson BD., Ii M., Park KW., Suli A., Sorensen LK., Larrieu-Lahargue F., Urness LD., Suh W., Asai J., Kock GA., Thorne T., Silver M., Thomas KR., Chien CB., Losordo DW., Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 313:640–644. 2006.
ArticleYebra M., Montgomery AM., Diaferia GR., Kaido T., Silletti S., Perez B., Just ML., Hildbrand S., Hurford R., Florkiewicz E., Tessier-Lavigne M., Cirulli V. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 5:695–707. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular matrix of the human retinal pigment epithelial cells in vitro
- Disulfiram, a Re-positioned Aldehyde Dehydrogenase Inhibitor, Enhances Radiosensitivity of Human Glioblastoma Cells In Vitro
- Netrin Inhibits Regenerative Axon Growth of Adult Dorsal Root Ganglion Neurons in Vitro
- Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells
- Cytomegalovirus-Specific Immunotherapy for Glioblastoma Treatments