J Vet Sci.
2013 Mar;14(1):53-60. 10.4142/jvs.2013.14.1.53.
Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine
- Affiliations
-
- 1College of Animal Science and Technology, Shanxi Agricultural University, Taigu 030801, China. yanfang6615@yahoo.com.cn
- 2Department of Pathology, the Second People's Hospital of Jinzhong, Taigu 030800, China.
- 3Department of Internal Neurology, the First People's Hospital of Jinzhong, Jinzhong 030600, China.
- KMID: 1482813
- DOI: http://doi.org/10.4142/jvs.2013.14.1.53
Abstract
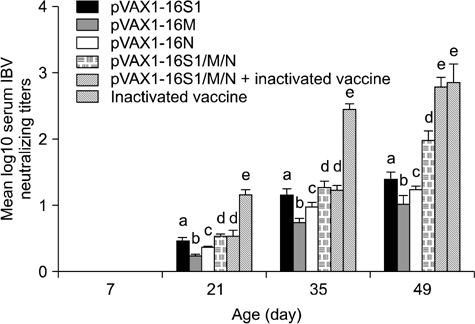
- The protective efficacy of DNA plasmids encoding avian infectious bronchitis virus (IBV) S1, N, or M protein was investigated in chickens. Chickens were inoculated monovalently (with plasmid pVAX1-16S1, pVAX1-16M, or pVAX1-16N alone) or multivalently (combination of the three different plasmids, pVAX1-16S1/M/N). A prime-boost immunization protocol against IBV was developed. Chickens were immunized with the multivalent DNA vaccine twice and then boosted with an inactivated vaccine once. Antibody titers of the chickens immunized with pVAX1-16S1/M/N were much higher than those of the monovalent groups (p < 0.01). A protective rate up to 90% was observed in the pVAX1-16S1/M/N group. The serum antibody titers in the prime-boost birds were significantly higher than those of the multivalent DNA vaccine group (p < 0.01) but not significantly different compared to the inactivated vaccine group at 49 days of age. Additionally, the prime-boost group also showed the highest level of IBV-specific cellular proliferation compared to the monovalent groups (p < 0.01) but no significant difference was found compared to the multivalent DNA vaccine group, and the prime-boost group completely protected from followed viral challenge.
Keyword
MeSH Terms
-
Aging
Animals
Antibodies, Viral/blood
Cell Proliferation
Chickens
Coronavirus Infections/prevention & control/*veterinary/virology
Immunization, Secondary/veterinary
Infectious bronchitis virus/*immunology
Poultry Diseases/*prevention & control/virology
T-Lymphocyte Subsets/cytology/physiology
Vaccines, DNA/immunology
Vaccines, Inactivated/immunology
Viral Vaccines/*immunology
Antibodies, Viral
Vaccines, DNA
Vaccines, Inactivated
Viral Vaccines
Figure
Reference
-
1. Barta O, Barta V, Pierson FW. Optimum conditions for the chicken lymphocyte transformation test. Avian Dis. 1992. 36:945–955.
Article2. Bijlenga G, Cook JKA, Gelb J Jr, de Wit JJ. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2004. 33:550–557.
Article3. Britton P, Cavanagh D. Thiel V, editor. Avian coronavirus diseases and infectious bronchitis virus vaccine development. Coronaviruses: Molecular and Cellular Biology. 2007. 1st ed. Norfolk: Caister Academic Press;161–181.4. Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003. 32:567–582.
Article5. Cavanagh D, Casais R, Armesto M, Hodgson T, Izadkhasti S, Davies M, Lin F, Tarpey I, Britton P. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine. 2007. 25:5558–5562.
Article6. Cavanagh D, Darbyshire JH, Davis P, Peters RW. Induction of humoral neutralising and haemagglutination-inhibiting antibody by the spike protein of avian infectious bronchitis virus. Avian Pathol. 1984. 13:573–583.
Article7. Farsang A, Ros C, Renström LHM, Baule C, Soós T, Belák S. Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathol. 2002. 31:229–236.
Article8. Ignjatovic J, Sapats S. Identification of previously unknown antigenic epitopes on the S and N proteins of avian infectious bronchitis virus. Arch Virol. 2005. 150:1813–1831.
Article9. Kapczynski DR, Hilt DA, Shapiro D, Sellers HS, Jackwood MW. Protection of chickens from infectious bronchitis by in ovo and intramuscular vaccination with a DNA vaccine expressing the S1 glycoprotein. Avian Dis. 2003. 47:272–285.
Article10. Larsen DL, Karasin A, Olsen CW. Immunization of pigs against influenza virus infection by DNA vaccine priming followed by killed-virus vaccine boosting. Vaccine. 2001. 19:2842–2853.
Article11. Lee CW, Hilt DA, Jackwood MW. Typing of field isolates of infectious bronchitis virus based on the sequence of the hypervariable region in the S1 gene. J Vet Diagn Invest. 2003. 15:344–348.
Article12. Lee EK, Jeon WJ, Lee YJ, Jeong OM, Choi JG, Kwon JH, Choi KS. Genetic diversity of avian infectious bronchitis virus isolates in Korea between 2003 and 2006. Avian Dis. 2008. 52:332–337.
Article13. Liu S, Wang Y, Ma Y, Han Z, Zhang Q, Shao Y, Chen J, Kong X. Identification of a newly isolated avian infectious bronchitis coronavirus variant in China exhibiting affinity for the respiratory tract. Avian Dis. 2008. 52:306–314.
Article14. Mase M, Tsukamoto K, Imai K, Yamaguchi S. Phylogenetic analysis of avian infectious bronchitis virus strains isolated in Japan. Arch Virol. 2004. 149:2069–2078.
Article15. McKinley ET, Hilt DA, Jackwood MW. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008. 26:1274–1284.
Article16. Pan Z, Zhang X, Geng S, Fang Q, You M, Zhang L, Jiao X, Liu X. Prime-boost immunization using a DNA vaccine delivered by attenuated Salmonella enterica serovar typhimurium and a killed vaccine completely protects chickens from H5N1 highly pathogenic avian influenza virus. Clin Vaccine Immunol. 2010. 17:518–523.
Article17. Pohuang T, Chansiripornchai N, Tawatsin A, Sasipreeyajan J. Detection and molecular characterization of infectious bronchitis virus isolated from recent outbreaks in broiler flocks in Thailand. J Vet Sci. 2009. 10:219–223.
Article18. Raj GD, Jones RC. Cross-reactive cellular immune responses in chickens vaccinated with live infectious bronchitis virus vaccine. Avian Pathol. 1997. 26:641–649.
Article19. Spaan W, Cavanagh D, Horzinek MC. Coronaviruses: structure and genome expression. J Gen Virol. 1988. 69(Pt 12):2939–2952.
Article20. Tang M, Wang H, Zhou S, Tian G. Enhancement of the immunogenicity of an infectious bronchitis virus DNA vaccine by a bicistronic plasmid encoding nucleocapsid protein and interleukin-2. J Virol Methods. 2008. 149:42–48.
Article21. Yan F, Yue W, Liu J, Li XY, Zhao YJ. Isolation and biological properties of avian infectious bronchitis virus isolated from Shanxi province. Chin J Vet Sci. 2009. 29:845–848.22. Yan F, Zhao Y, Wu Q, Yue W, Li X, Ji W, Liu J, Liu F, Ren J, Hua L. Cloning and expression of the S1, N and M gene of IBV. Zhongguo Nong Xue Tong Bao. 2010. 26:14–17.23. Yan F, Zhao Y, Yue W, Yao J, Lihua L, Ji W, Li X, Liu F, Wu Q. Phylogenetic analysis of S1 gene of infectious bronchitis virus isolates from China. Avian Dis. 2011. 55:451–458.
Article24. Yang T, Wang HN, Wang X, Tang JN, Gao R, Li J, Guo ZC, Li YL. Multivalent DNA vaccine enhanced protection efficacy against infectious bronchitis virus in chickens. J Vet Med Sci. 2009. 71:1585–1590.
Article25. Yin Z, Liu J. Animal Virology. 1997. 2nd ed. Beijing: Science Press;329–331.26. Zou NL, Zhao FF, Wang YP, Liu P, Cao SJ, Wen XT, Huang Y. Genetic analysis revealed LX4 genotype strains of avian infectious bronchitis virus became predominant in recent years in Sichuan area, China. Virus Genes. 2010. 41:202–209.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of an attenuated vaccine strain from a korean respiratory type infectious bronchitis virus
- Improved immunogenicity of Newcastle disease virus inactivated vaccine following DNA vaccination using Newcastle disease virus hemagglutinin-neuraminidase and fusion protein genes
- Effects of DDA, CpG-ODN, and plasmid-encoded chicken IFN-gamma on protective immunity by a DNA vaccine against IBDV in chickens
- Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-gamma
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology