J Vet Sci.
2013 Mar;14(1):15-20. 10.4142/jvs.2013.14.1.15.
Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels
- Affiliations
-
- 1Department of Theriogenology and Biotechnology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. bclee@snu.ac.kr
- 2Institute of Green Bio Science and Technology, Seoul National University, Pyeongchang 232-916, Korea.
- KMID: 1482808
- DOI: http://doi.org/10.4142/jvs.2013.14.1.15
Abstract
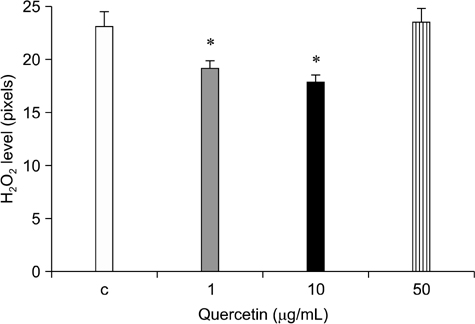
- Quercetin is a plant-derived flavonoid found in fruits or vegetables that has antioxidant properties and acts as a free radical scavenger. We investigated the effects of quercetin on porcine oocyte nuclear maturation and embryonic development after parthenogenetic activation. We then evaluated the antioxidant activities of quercetin by measuring reactive oxygen species (ROS) levels in matured oocytes. Immature oocytes were untreated or treated with 1, 10, and 50 microg/mL quercetin during in vitro maturation (IVM). Quercetin treatment did not improve oocyte nuclear maturation, but significantly higher blastocyst rates (p < 0.05) of parthenogenetically activated oocytes were achieved when the IVM medium was supplemented with an adequate concentration of quercetin (1 microg/mL). However, cleavage rates and blastocyst cell numbers were not affected. Oocytes treated with 1 or 10 microg/mL quercetin had significantly lower (p < 0.05) levels of ROS than the control and group treated with the highest concentration of quercetin (50 microg/mL). Moreover, this highest concentration was detrimental to oocyte nuclear maturation and blastocyst formation. Based on our findings, we concluded that exogenous quercetin reduces ROS levels during oocyte maturation and is beneficial for subsequent embryo development.
Keyword
MeSH Terms
-
Animals
Antioxidants/administration & dosage/*pharmacology
Dose-Response Relationship, Drug
In Vitro Oocyte Maturation Techniques/*veterinary
Oocytes/cytology/*drug effects/physiology
Quercetin/administration & dosage/*pharmacology
Reactive Oxygen Species/*metabolism
*Swine
Antioxidants
Reactive Oxygen Species
Quercetin
Figure
Reference
-
1. Ader P, Wessmann A, Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic Biol Med. 2000. 28:1056–1067.
Article2. Barcelos GRM, Angeli JPF, Serpeloni JM, Grotto D, Rocha BA, Bastos JK, Knasmüller S, Júnior FB. Quercetin protects human-derived liver cells against mercury-induced DNA-damage and alterations of the redox status. Mutat Res. 2011. 726:109–115.
Article3. Boots AW, Li H, Schins RPF, Duffin R, Heemskerk JWM, Bast A, Haenen GRMM. The quercetin paradox. Toxicol Appl Pharmacol. 2007. 222:89–96.
Article4. Chan WH. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum Reprod. 2006. 21:2985–2995.
Article5. Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod Toxicol. 2009. 28:52–58.
Article6. Chen CC, Chan WH. Impact effects of puerarin on mouse embryonic development. Reprod Toxicol. 2009. 28:530–535.
Article7. Dalvit G, Llanes SP, Descalzo A, Insani M, Beconi M, Cetica P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod Domest Anim. 2005. 40:93–97.
Article8. Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009. 296:R1071–R1077.
Article9. De La Fuente R, King WA. Developmental consequences of karyokinesis without cytokinesis during the first mitotic cell cycle of bovine parthenotes. Biol Reprod. 1998. 58:952–962.10. Dusza L, Ciereszko R, Skarzyński DJ, Nogowski L, Opałka M, Kamińska B, Nynca A, Kraszewska O, Słomczyńska M, Woclawek-Potocka I, Korzekwa A, Pruszyńska-Oszmałek E, Szkudelska K. Mechanism of phytoestrogen action in reproductive processes of mammals and birds. Reprod Biol. 2006. 6:Suppl 1. 151–174.11. Gargouri B, Mansour RB, Abdallah FB, Elfekih A, Lassoued S, Khaled H. Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids Health Dis. 2011. 10:149.
Article12. Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993. 15:69–75.
Article13. Graefe EU, Derendorf H, Veit M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int J Clin Pharmacol Ther. 1999. 37:219–233.14. Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001. 7:175–189.
Article15. Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002. 123:479–486.
Article16. Iwamoto M, Onishi A, Fuchimoto D, Somfai T, Takeda K, Tagami T, Hanada H, Noguchi J, Kaneko H, Nagai T, Kikuchi K. Low oxygen tension during in vitro maturation of porcine follicular oocytes improves parthenogenetic activation and subsequent development to the blastocyst stage. Theriogenology. 2005. 63:1277–1289.
Article17. Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res. 2006. 55:168–175.
Article18. Kang JT, Atikuzzaman M, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC. Developmental competence of porcine oocytes after in vitro maturation and in vitro culture under different oxygen concentrations. Zygote. 2012. 20:1–8.
Article19. Kang JT, Koo OJ, Kwon DK, Park HJ, Jang G, Kang SK, Lee BC. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009. 46:22–28.
Article20. Karja NW, Wongsrikeao P, Murakami M, Agung B, Fahrudin M, Nagai T, Otoi T. Effects of oxygen tension on the development and quality of porcine in vitro fertilized embryos. Theriogenology. 2004. 62:1585–1595.
Article21. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004. 62:1186–1197.
Article22. Liang W, Li X, Li C, Liao L, Gao B, Gan H, Yang Z, Liao L, Chen X. Quercetin-mediated apoptosis via activation of the mitochondrial-dependent pathway in MG-63 osteosarcoma cells. Mol Med Rep. 2011. 4:1017–1023.23. Naderi GA, Asgary S, Sarraf-Zadegan N, Shirvany H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol Cell Biochem. 2003. 246:193–196.
Article24. Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990. 109:501–507.
Article25. Park MH, Min DS. Quercetin-induced downregulation of phospholipase D1 inhibits proliferation and invasion in U87 glioma cells. Biochem Biophys Res Commun. 2011. 412:710–715.
Article26. Sabri A, Hughie HH, Lucchesi PA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal. 2003. 5:731–740.
Article27. Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev. 2007. 53:605–614.
Article28. Santini SE, Basini G, Bussolati S, Grasselli F. The phytoestrogen quercetin impairs steroidogenesis and angiogenesis in swine granulosa cells in vitro. J Biomed Biotechnol. 2009. 2009:419891.
Article29. Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-α-glucoside during in vitro maturation. Biol Reprod. 2001. 65:1800–1806.
Article30. Uhm SJ, Gupta MK, Yang JH, Lee SH, Lee HT. Selenium improves the developmental ability and reduces the apoptosis in porcine parthenotes. Mol Reprod Dev. 2007. 74:1386–1394.
Article31. You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology. 2010. 74:777–785.
Article32. Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006. 12:3193–3199.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Relationship Between Spermatozoa Ability and Reactive Oxygen Species in Porcine: I. Sperm Preincubation by Xanthine and Xanthine Oxidase
- Antioxidant effect of ergothioneine on in vitro maturation of porcine oocytes
- Follicular fluid-derived extracellular vesicles improve in vitro maturation and embryonic development of porcine oocytes
- Do Reactive Oxygen Species Cause Aging?
- Activation of Porcine Oocytes Following Intracytoplasmic Injection of Various Sperm Components and foreign species spermatozoa