J Vet Sci.
2013 Mar;14(1):1-6. 10.4142/jvs.2013.14.1.1.
C6, a new monoclonal antibody, reacts with the follicle-associated epithelium of calf ileal Peyer's patches
- Affiliations
-
- 1Department of Veterinary Anatomy, Faculty of Agriculture, University of Miyazaki, Miyazaki 889-2192, Japan. yasudaja@cc.miyazaki-u.ac.jp
- 2Department of Veterinary Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 3AgResearch, The Hopkirk Research Institute, Grasslands Research Centre, Palmerston North 4442, New Zealand.
- KMID: 1482806
- DOI: http://doi.org/10.4142/jvs.2013.14.1.1
Abstract
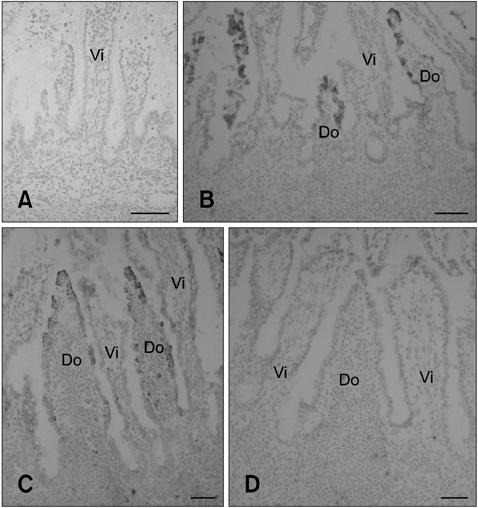
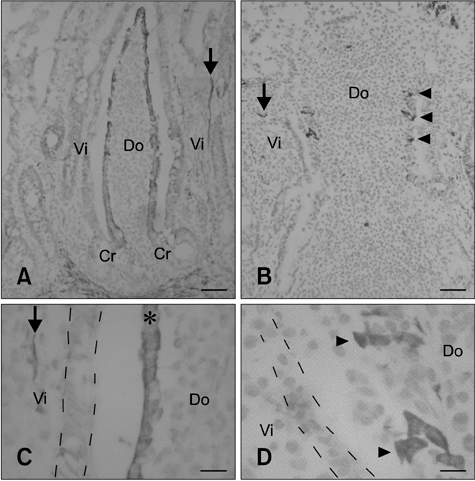
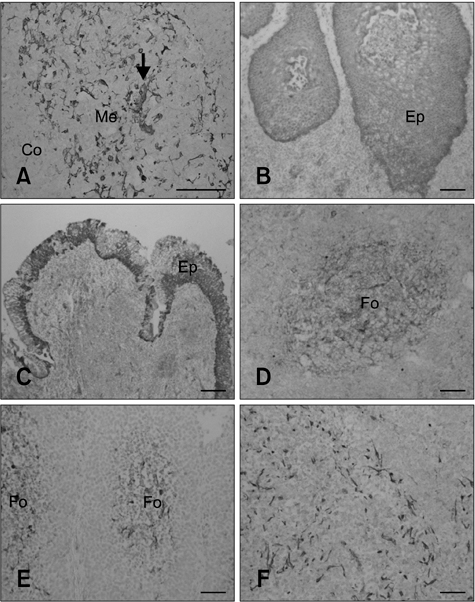
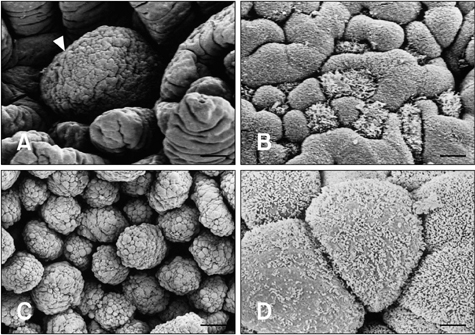
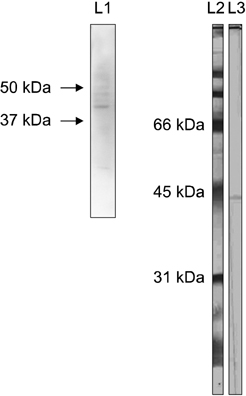
- The follicle-associated epithelium (FAE) of Peyer's patches (PPs) contains M cells that are important for reducing mucosal immune responses by transporting antigens into the underlying lymphoid tissue. We generated a monoclonal antibody (C6) that reacted with the FAE of calf ileal PPs, and analyzed the characteristics of C6 using immunohistochemistry and Western blotting. FAE of the ileal PP was stained with C6 during both late fetal developmental and postnatal stages. Neither the villous epithelial cell nor intestinal crypt basal cells were stained at any developmental stage. During the prenatal stages, FAE of the jejunal PP was C6-negative. However, a few C6-positive cells were distributed diffusely in some FAE of the jejunal PPs during the postnatal stages. The protein molecular weight of the antigen recognized by C6 was approximately 45 kDa. These data show that C6 is useful for identifying the FAE in ileal PPs and further suggest that differentiation of the FAE in these areas is independent of external antigens.
MeSH Terms
Figure
Reference
-
1. Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991. 4:760–765.2. Anderle P, Rumbo M, Sierro F, Mansourian R, Michetti P, Roberts MA, Kraehenbuhl JP. Novel markers of the human follicle-associated epithelium identified by genomic profiling and microdissection. Gastroenterology. 2005. 129:321–327.
Article3. Asari M, Kano Y, Wakui S, Nishita T, Matsushita H, Oshige H. The ultrastructure of bovine ileal follicle-associated epithelial (FAE) cells during the perinatal period. J Anat. 1989. 163:7–16.4. Azzali G. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol Rev. 2003. 195:178–189.
Article5. Beyaz F, Aşti RN. Development of ileal Peyer's patches and follicle associated epithelium in bovine foetuses. Anat Histol Embryol. 2004. 33:172–179.
Article6. Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993. 41:1679–1687.
Article7. Debard N, Sierro F, Browning J, Kraehenbuhl JP. Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer's patches. Gastroenterology. 2001. 120:1173–1182.
Article8. Gebert A, Hach G, Bartels H. Co-localization of vimentin and cytokeratins in M-cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res. 1992. 269:331–340.
Article9. Gebert A, Rothkötter HJ, Pabst R. Cytokeratin 18 is an M-cell marker in porcine Peyer's patches. Cell Tissue Res. 1994. 276:213–221.
Article10. Heggebø R, Press CM, Gunnes G, Lie KI, Tranulis MA, Ulvund M, Groschup MH, Landsverk T. Distribution of prion protein in the ileal Peyer's patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J Gen Virol. 2000. 81:2327–2337.
Article11. Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A. Transepithelial prion transport by M cells. Nat Med. 2001. 7:976–977.
Article12. Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, Hiroi T, Tamagawa H, Iijima H, Kunisawa J, Yuki Y, Kiyono H. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004. 101:6110–6115.
Article13. Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH. Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J. 1992. 24:33–39.
Article14. Jepson MA, Simmons NL, Hirst GL, Hirst BH. Identification of M cells and their distribution in rabbit intestinal Peyer's patches and appendix. Cell Tissue Res. 1993. 273:127–136.
Article15. Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994. 180:15–23.
Article16. Kanaya T, Aso H, Miyazawa K, Kido T, Minashima T, Watanabe K, Ohwada S, Kitazawa H, Rose MT, Yamaguchi T. Staining patterns for actin and villin distinguish M cells in bovine follicle-associated epithelium. Res Vet Sci. 2007. 82:141–149.
Article17. Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997. 277:949–952.
Article18. Landsverk T. Phagocytosis and transcytosis by the follicle-associated epithelium of the ileal Peyer's patch in calves. Immunol Cell Biol. 1988. 66(Pt 4):261–268.
Article19. Landsverk T. The follicle-associated epithelium of the ileal Peyer's patch in ruminants is distinguished by its shedding of 50 nm particles. Immunol Cell Biol. 1987. 65(Pt 3):251–261.
Article20. Lie KI, Press CM, McCullagh P, McClure SJ, Landsverk T. Differentiation of the follicle-associated epithelium in ileal Peyer's patch and production of 50-nm particles are maintained in B-cell-depleted fetal sheep. Cell Tissue Res. 2005. 319:395–404.
Article21. Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005. 206:177–189.
Article22. Parsons KR, Bland AP, Hall GA. Follicle associated epithelium of the gut associated lymphoid tissue of cattle. Vet Pathol. 1991. 28:22–29.
Article23. Pernthaner A, Cole SA, Gatehouse T, Hein WR. Phenotypic diversity of antigen-presenting cells in ovine-afferent intestinal lymph. Arch Med Res. 2002. 33:405–412.
Article24. Press CM, Reynolds JD, McClure SJ, Landsverk T. Development of accessory cells in B-cell compartments is retarded in B-cell-depleted fetal sheep. Dev Immunol. 1998. 6:223–231.
Article25. Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA. Immunocytochemical characterization of the follicle-associated epithelium of Peyer's patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol. 1996. 71:363–370.26. Rumbo M, Sierro F, Debard N, Kraehenbuhl JP, Finke D. Lymphotoxin β receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004. 127:213–223.
Article27. Shmakov AN, Ghosh S. Prion proteins and the gut: une liaison dangereuse? Gut. 2001. 48:443–447.
Article28. Tanaka Y, Imai T, Baba M, Ishikawa I, Uehira M, Nomiyama H, Yoshie O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999. 29:633–642.
Article29. Yasuda M, Fujino M, Nasu T, Murakami T. Histological studies on the ontogeny of bovine gut-associated lymphoid tissue: appearance of T cells and development of IgG+ and IgA+ cells in lymphoid follicles. Dev Comp Immunol. 2004. 28:357–369.
Article30. Yasuda M, Tanaka S, Arakawa H, Taura Y, Yokomizo Y, Ekino S. A comparative study of gut-associated lymphoid tissue in calf and chicken. Anat Rec. 2002. 266:207–217.
Article31. Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, Iwasaki A. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer's patch CD11b+ dendritic cells. J Immunol. 2003. 171:2797–2803.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intestinal Peyer’s Patches: Structure, Function, and In Vitro Modeling
- Can narrow-band imaging of Peyer's patches predict the recurrence of ulcerative colitis?
- Ultrastructural Changes of Epithelium Covering Peyer's Patch by Simple Obstruction of Gerbil Ileum
- Diagnosis of bovine cryptosporidiosis by indirect immunofluorescence assay using monoclonal antibody
- Role of IGF2 Gene in Developing Human Ovary