Korean J Radiol.
2013 Apr;14(2):248-258. 10.3348/kjr.2013.14.2.248.
Radiofrequency Ablation Combined with Chemoembolization for Intermediate-Sized (3-5 cm) Hepatocellular Carcinomas Under Dual Guidance of Biplane Fluoroscopy and Ultrasonography
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. leeminwoo0@gmail.com
- 2Department of Radiology, Kangwon National University College of Medicine, Chuncheon 200-722, Korea.
- KMID: 1482784
- DOI: http://doi.org/10.3348/kjr.2013.14.2.248
Abstract
OBJECTIVE
To assess the technical feasibility and local efficacy of percutaneous radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) for an intermediate-sized (3-5 cm in diameter) hepatocellular carcinoma (HCC) under the dual guidance of biplane fluoroscopy and ultrasonography (US).
MATERIALS AND METHODS
Patients with intermediate-sized HCCs were treated with percutaneous RFA combined with TACE. RFA was performed under the dual guidance of biplane fluoroscopy and US within 14 days after TACE. We evaluated the rate of major complications on immediate post-RFA CT images. Primary technique effectiveness rate was determined on one month follow-up CT images. The cumulative rate of local tumor progression was estimated with the use of Kaplan-Meier method.
RESULTS
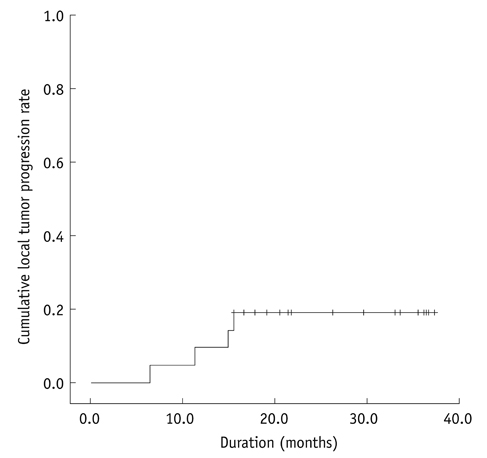
Twenty-one consecutive patients with 21 HCCs (mean size: 3.6 cm; range: 3-4.5 cm) were included. After TACE (mean: 6.7 d; range: 1-14 d), 20 (95.2%) of 21 HCCs were visible on fluoroscopy and were ablated under dual guidance of biplane fluoroscopy and US. The other HCC that was poorly visible by fluoroscopy was ablated under US guidance alone. Major complications were observed in only one patient (pneumothorax). Primary technique effectiveness was achieved for all 21 HCCs in a single RFA session. Cumulative rates of local tumor progression were estimated as 9.5% and 19.0% at one and three years, respectively.
CONCLUSION
RFA combined with TACE under dual guidance of biplane fluoroscopy and US is technically feasible and effective for intermediate-sized HCC treatment.
Keyword
MeSH Terms
-
Aged
Antibiotics, Antineoplastic/administration & dosage
Antineoplastic Agents/administration & dosage
Carcinoma, Hepatocellular/*drug therapy/radiography/*surgery/ultrasonography
Catheter Ablation/*methods
Chemoembolization, Therapeutic/*methods
Combined Modality Therapy
Disease Progression
Doxorubicin/administration & dosage
Ethiodized Oil/administration & dosage
Feasibility Studies
Female
Fluoroscopy
Humans
Liver Neoplasms/*drug therapy/radiography/*surgery/ultrasonography
Male
Postoperative Complications
*Radiography, Interventional
Retrospective Studies
Tomography, X-Ray Computed
Treatment Outcome
*Ultrasonography, Interventional
Antibiotics, Antineoplastic
Antineoplastic Agents
Doxorubicin
Ethiodized Oil
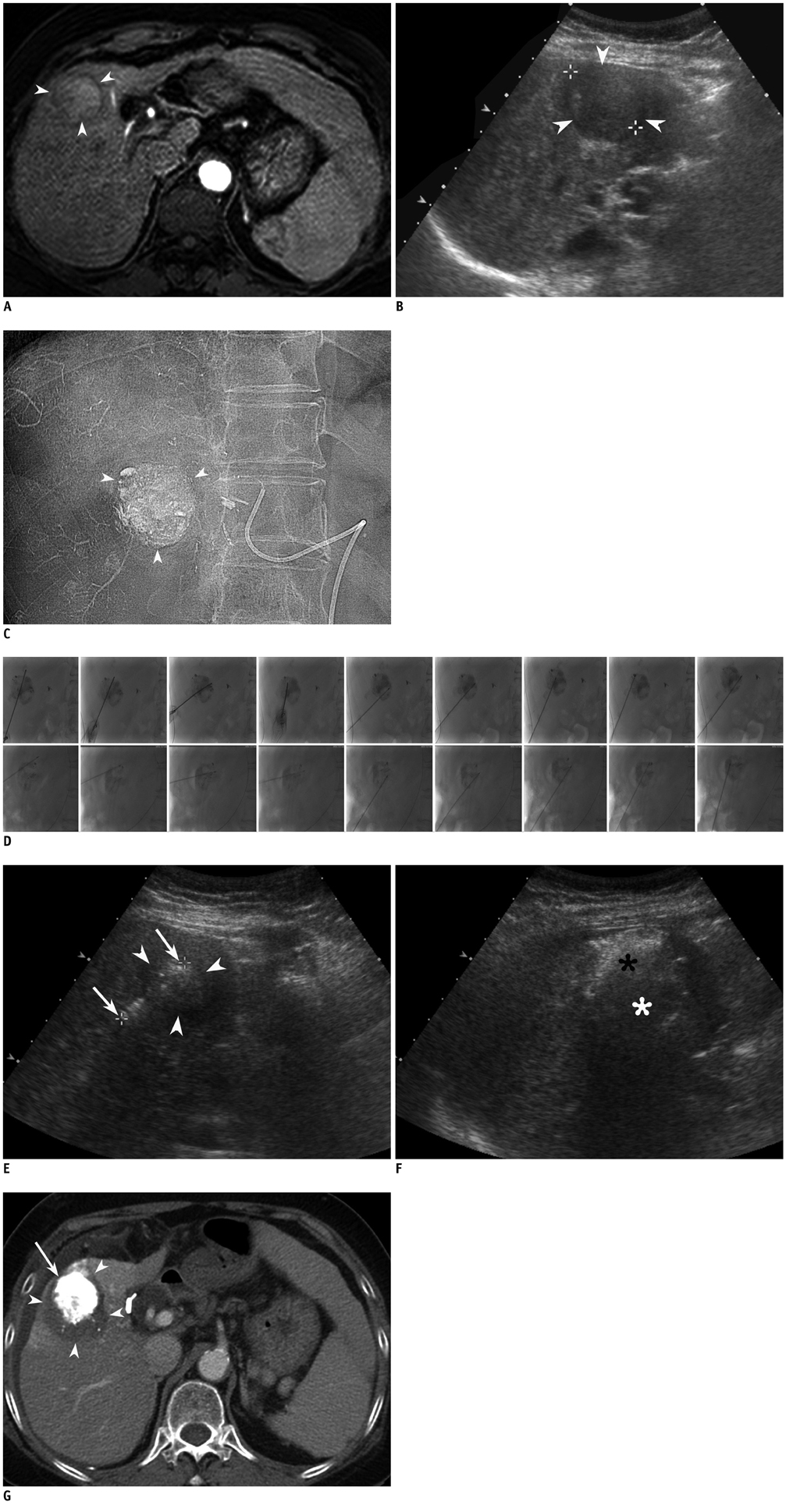
Figure
Cited by 1 articles
-
Recent advance in international management of hepatocellular carcinoma
Jin Wook Chung
J Korean Med Assoc. 2013;56(11):972-982. doi: 10.5124/jkma.2013.56.11.972.
Reference
-
1. Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011. 53:136–147.2. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008. 47:82–89.3. Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008. 48:Suppl 1. S20–S37.4. Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005. 103:299–306.5. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011. 53:1020–1022.6. Kudo M, Okanoue T. Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007. 72:Suppl 1. 2–15.7. Takaki H, Yamakado K, Nakatsuka A, Fuke H, Murata K, Shiraki K, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol. 2007. 18:856–861.8. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008. 247:260–266.9. Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011. 18:1624–1629.10. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010. 116:5452–5460.11. Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998. 9(1 Pt 1):101–111.12. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.13. Gasparini D, Sponza M, Marzio A, Zanardi R, Bazzocchi M, Cemal Y. Combined treatment, TACE and RF ablation, in HCC: preliminary results. Radiol Med. 2002. 104:412–420.14. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.15. Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, et al. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011. 84:691–697.16. Yamakado K, Nakatsuka A, Takaki H, Sakurai H, Isaji S, Yamamoto N, et al. Subphrenic versus nonsubphrenic hepatocellular carcinoma: combined therapy with chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2010. 194:530–535.17. Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009. 20:1570–1577.18. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.19. Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010. 75:253–258.20. Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009. 19:2630–2640.21. Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009. 34:371–380.22. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009. 252:905–913.23. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009. 20:7 Suppl. S377–S390.24. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010. 194:W396–W400.25. Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009. 10:34–42.26. Lee MW, Kim YJ, Park SW, Yu NC, Park HS, Jung SI, et al. Sequential changes in echogenicity and conspicuity of small hepatocellular carcinoma on gray scale sonography after transcatheter arterial chemoembolization. J Ultrasound Med. 2010. 29:1305–1312.27. Shibata T, Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004. 15:1323–1327.28. Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006. 12:608–611.29. Kato T, Yamagami T, Hirota T, Matsumoto T, Yoshimatsu R, Nishimura T. Transpulmonary radiofrequency ablation for hepatocellular carcinoma under real-time computed tomography-fluoroscopic guidance. Hepatogastroenterology. 2008. 55:1450–1453.30. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012. 13:34–43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiofrequency Ablation for Viable Hepatocellular Carcinoma around Retained Iodized Oil after Transcatheter Arterial Chemoembolization: Usefulness of Biplane Fluoroscopy Plus Ultrasound Guidance
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?
- Current status and future of radiofrequency ablation for hepatocellular carcinoma
- Radiofrequency Ablation for Hepatocellular Carcinoma
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment