Immune Netw.
2009 Oct;9(5):179-191. 10.4110/in.2009.9.5.179.
Prophylactic and Therapeutic Potential of Asp f1 Epitopes in Naive and Sensitized BALB/c Mice
- Affiliations
-
- 1Institute of Genomics and Integrative Biology, Mall Road, Delhi, India. taruna_m@hotmail.com
- 2School of Biotechnology, Devi Ahilya Vishwavidyalaya, Khandwa Road, Indore, India.
- 3Institute of Microbial Technology, Sector-39A, Chandigarh, India.
- 4Biopolymers Division, Central Drug Research Institute, Lucknow, India.
- KMID: 1474579
- DOI: http://doi.org/10.4110/in.2009.9.5.179
Abstract
- BACKGROUND
The present study examines a hypothesis that short allergen-derived peptides may shift an Aspergillus fumigatus (Afu-) specific TH2 response towards a protective TH1. Five overlapping peptides (P1-P5) derived from Asp f1, a major allergen/antigen of Afu, were evaluated for prophylactic or therapeutic efficacy in BALB/c mice.
METHODS
To evaluate the prophylactic efficacy, peptides were intranasally administered to naive mice and challenged with Afu-allergens/antigens. For evaluation of therapeutic efficacy, the mice were sensitized with Afu-allergens/antigens followed by intranasal administration of peptides. The groups were compared for the levels of Afu-specific antibodies in sera and splenic cytokines evaluated by ELISA. Eosinophil peroxidase activity was examined in the lung cell suspensions and lung inflammation was assessed by histopathogy.
RESULTS
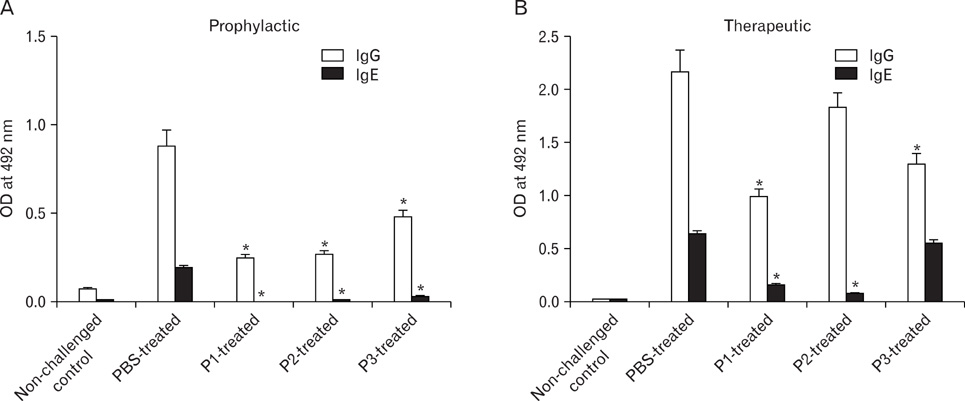
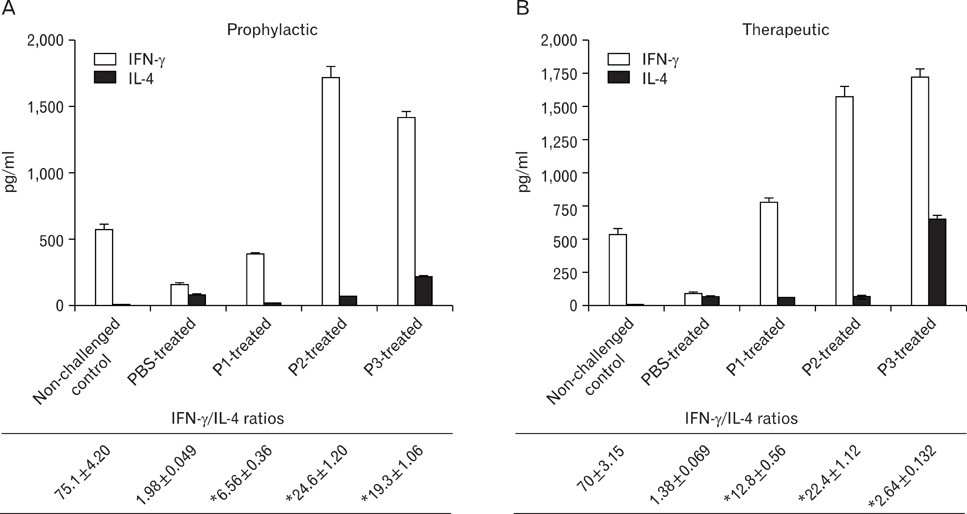
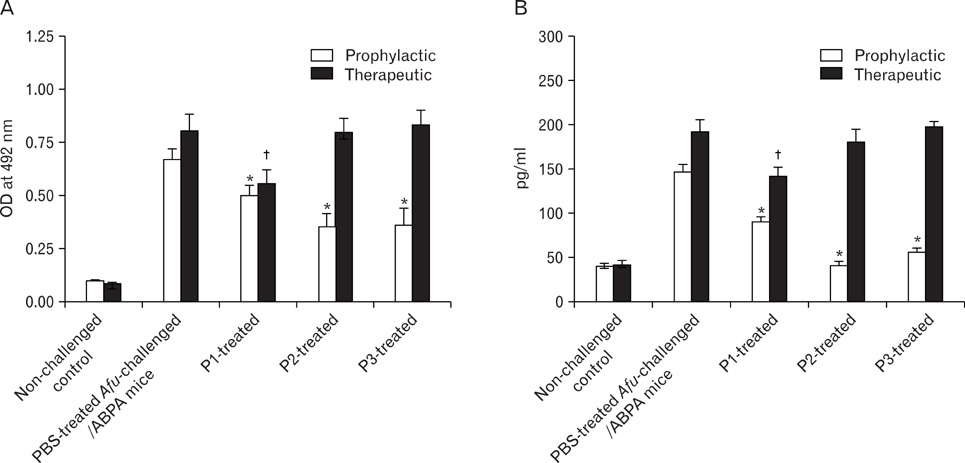
Peptides P1-, P2- and P3 decreased Afu-specific IgE (84.5~98.9%) and IgG antibodies (45.7~71.6%) in comparison with Afu-sensitized mice prophylactically. P1- and P2-treated ABPA mice showed decline in Afu-specific IgE (76.4~88%) and IgG antibodies (15~54%). Increased IgG2a/IgG1 and IFN-gamma/IL-4 ratios were observed. P1-P3 prophylactically and P1 therapeutically decreased IL-5 levels and eosinophil peroxidase activity. P1 decreased inflammatory cells' infiltration in lung tissue comparable to non-challenged control.
CONCLUSION
Asp f1-derived peptide P1, prophylactically and therapeutically administered to Balb/c mice, is effective in regulating allergic response to allergens/antigens of Afu, and may be explored for immunotherapy of allergic aspergillosis in humans.
Keyword
MeSH Terms
-
Administration, Intranasal
Animals
Antibodies
Aspergillosis
Aspergillosis, Allergic Bronchopulmonary
Aspergillus fumigatus
Cytokines
Enzyme-Linked Immunosorbent Assay
Eosinophil Peroxidase
Eosinophils
Epitopes
Humans
Immunoglobulin E
Immunoglobulin G
Immunotherapy
Interleukin-5
Lung
Mice
Peptides
Pneumonia
Suspensions
Viperidae
Antibodies
Cytokines
Eosinophil Peroxidase
Epitopes
Immunoglobulin E
Immunoglobulin G
Interleukin-5
Peptides
Suspensions
Figure
Reference
-
1. Tillie-Leblond I, Tonnel AB. Allergic bronchopulmonary aspergillosis. Allergy. 2005. 60:1004–1013.
Article2. Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, Denning DW, Crameri R, Brody AS, Light M, Skov M, Maish W, Mastella G. Participants in the Cystic Fibrosis Foundation Consensus Conference: Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003. 37:Suppl 3. S225–S264.3. Zander DS. Allergic bronchopulmonary aspergillosis: an overview. Arch Pathol Lab Med. 2005. 129:924–928.
Article4. Eaton T, Garrett J, Milne D, Frankel A, Wells AU. Allergic bronchopulmonary aspergillosis in the asthma clinic. A prospective evaluation of CT in the diagnostic algorithm. Chest. 2000. 118:66–72.
Article5. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006. 6:761–771.
Article6. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007. 119:780–791.
Article7. Ajduk J, Marinic I, Aberle N, Rabatic S, Gagro A. Effect of house dust mite immunotherapy on transforming growth factor beta1-producing T cells in asthmatic children. Ann Allergy Asthma Immunol. 2008. 100:314–323.8. Pfaar O, Klimek L. Anaphylaxis--life-threatening allergic reactions. MMW Fortschr Med. 2008. 150:35–39.9. Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008. 100:256–263.
Article10. Senti G, Johansen P, Martinez Gomez J, Prinz Varicka BM, Kündig TM. Efficacy and safety of allergen-specific immunotherapy in rhinitis, rhinoconjunctivitis, and bee/wasp venom allergies. Int Rev Immunol. 2005. 24:519–531.
Article11. Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009. 123:735–746.
Article12. Lizaso MT, Tabar AI, García BE, Gómez B, Algorta J, Asturias JA, Martinez A. Double-blind, placebo-controlled Alternaria alternata immunotherapy: in vivo and in vitro parameters. Pediatr Allergy Immunol. 2008. 19:76–81.13. Malling HJ, Djurup R. Diagnosis and immunotherapy of mould allergy. VII. IgG subclass response and relation to the clinical efficacy of immunotherapy with Cladosporium. Allergy. 1988. 43:60–70.14. Tabar AI, Lizaso MT, García BE, Gómez B, Echechipía S, Aldunate MT, Madariaga B, Martínez A. Double-blind, placebo-controlled study of Alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. 2008. 19:67–75.
Article15. Chauhan B, Knutsen A, Hutcheson PS, Slavin RG, Bellone CJ. T cell subsets, epitope mapping, and HLA-restriction in patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 1996. 97:2324–2331.
Article16. Kurup VP, Banerjee B, Murali PS, Greenberger PA, Krishnan M, Hari V, Fink JN. Immunodominant peptide epitopes of allergen, Asp f1 from the fungus Aspergillus fumigatus. Peptides. 1998. 19:1469–1477.
Article17. Madan T, Arora N, Sarma PU. Ribonuclease activity dependent cytotoxicity of Asp fl, a major allergen of A. fumigatus. Mol Cell Biochem. 1997. 175:21–27.18. Madan T, Arora N, Sarma PU. Identification and evaluation of a major cytotoxin of A. fumigatus. Mol Cell Biochem. 1997. 167:89–97.19. Madan T, Priyadarsiny P, Vaid M, Kamal N, Shah A, Haq W, Katti SB, Sarma PU. Use of a synthetic peptide epitope of Asp f1, a major allergen or antigen of Aspergillus fumigatus, for improved immunodiagnosis of allergic bronchopulmonary aspergillosis. Clin Diagn Lab Immunol. 2004. 11:552–558.
Article20. Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of cytotoxic T cell responses directed to human leucocyte antigen Class I restricted epitopes from the Aspergillus f16 allergen. Clin Exp Immunol. 2005. 140:81–91.
Article21. Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005. 139:257–267.
Article22. Svirshchevskaya EV, Alekseeva LG, Titov VM, Frolova EG, Sapozhnikov AM. Determination of T and B Cell Epitopes of Aspergillus fumigatus Ribotoxin and Heat Shock Protein. Russ J Immunol. 1998. 3:61–68.23. Arruda LK, Mann BJ, Chapman MD. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992. 149:3354–3359.24. Moser M, Crameri R, Menz G, Schneider T, Dudler T, Virchow C, Gmachl M, Blaser K, Suter M. Cloning and expression of recombinant Aspergillus fumigatus allergen I/a (rAsp f I/a) with IgE binding and type I skin test activity. J Immunol. 1992. 149:454–460.25. Kamal N, Chowdhury S, Madan T, Sharma D, Attreyi M, Haq W, Katti SB, Kumar A, Sarma PU. Tryptophan residue is essential for immunoreactivity of a diagnostically relevant peptide epitope of A. fumigatus. Mol Cell Biochem. 2005. 275:223–231.
Article26. Svirshchevskaya E, Frolova E, Alekseeva L, Kotzareva O, Kurup VP. Intravenous injection of major and cryptic peptide epitopes of ribotoxin, Asp f1 inhibits T cell response induced by crude Aspergillus fumigatus antigens in mice. Peptides. 2000. 21:1–8.
Article27. Bhasin M, Raghava GP. SVM based method for predicting HLA-DRB1*0401 binding peptides in an antigen sequence. Bioinformatics. 2004. 20:421–423.
Article28. Zhang GL, Srinivasan KN, Veeramani A, August JT, Brusic V. PREDBALB/c: a system for the prediction of peptide binding to H2d molecules, a haplotype of the BALB/c mouse. Nucleic Acids Res. 2005. 33:W180–W183.
Article29. Bhasin M, Raghava GP. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine. 2004. 22:3195–3204.
Article30. Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, Reid KB, Sarma PU. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001. 107:467–475.
Article31. Astori M, von Garnier C, Kettner A, Dufour N, Corradin G, Spertini F. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J mice. J Immunol. 2000. 165:3497–3505.
Article32. Hall G, Houghton CG, Rahbek JU, Lamb JR, Jarman ER. Suppression of allergen reactive Th2 mediated responses and pulmonary eosinophilia by intranasal administration of an immunodominant peptide is linked to IL-10 production. Vaccine. 2003. 21:549–561.
Article33. Nigam S, Ghosh PC, Sarma PU. Altered immune response to liposomal allergens of Aspergillus fumigatus in mice. Int J Pharm. 2002. 236:97–109.
Article34. Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968. 97:77–89.35. Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005. 35:52–58.
Article36. Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993. 178:1783–1788.
Article37. Kay AB, Larché M. Allergen immunotherapy with cat allergen peptides. Springer Semin Immunopathol. 2004. 25:391–399.
Article38. Yoshitomi T, Nakagami Y, Hirahara K, Taniguchi Y, Sakaguchi M, Yamashita M. Intraoral administration of a T-cell epitope peptide induces immunological tolerance in Cry j 2-sensitized mice. J Pept Sci. 2007. 13:499–503.
Article39. Helbling A, Reimers A. Immunotherapy in fungal allergy. Curr Allergy Asthma Rep. 2003. 3:447–453.
Article40. Crameri R, Weichel M, Flückiger S, Glaser AG, Rhyner C. Fungal allergies: a yet unsolved problem. Chem Immunol Allergy. 2006. 91:121–133.
Article41. Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008. 145:58–86.
Article42. Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988. 140:1022–1027.43. Kozutsumi D, Tsunematsu M, Yamaji T, Murakami R, Yokoyama M, Kino K. Cry-consensus peptide, a novel peptide for immunotherapy of Japanese cedar pollinosis, induces Th1-predominant response in Cry j 1-sensitized B10.S mice. Biol Pharm Bull. 2006. 29:2506–2509.
Article44. von Garnier C, Astori M, Kettner A, Dufour N, Heusser C, Corradin G, Spertini F. Allergen-derived long peptide immunotherapy down-regulates specific IgE response and protects from anaphylaxis. Eur J Immunol. 2000. 30:1638–1645.
Article45. Furin MJ, Norman PS, Creticos PS, Proud D, Kagey-Sobotka A, Lichtenstein LM, Naclerio RM. Immunotherapy decreases antigen-induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991. 88:27–32.
Article46. Rak S, Löwhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol. 1988. 82:470–480.
Article47. Hamelmann E, Wahn U, Gelfand EW. Role of the Th2 cytokines in the development of allergen-induced airway inflammation and hyperresponsiveness. Int Arch Allergy Immunol. 1999. 118:90–94.
Article48. Kurup VP, Murali PS, Guo J, Choi H, Banerjee B, Fink JN, Coffman RL. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy. 1997. 52:1215–1221.
Article49. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004. 4:1–23.
Article50. Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005. 201:169–171.51. Jutel M, Klunker S, Akdis M, Malolepszy J, Thomet OA, Zak-Nejmark T, Blaser K, Akdis CA. Histamine upregulates Th1 and downregulates Th2 responses due to different patterns of surface histamine 1 and 2 receptor expression. Int Arch Allergy Immunol. 2001. 124:190–192.
Article52. Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001. 413:420–425.
Article53. Johansen P, Senti G, Maria Martínez Gómez J, Kündig TM. Medication with antihistamines impairs allergen-specific immunotherapy in mice. Clin Exp Allergy. 2008. 38:512–519.
Article54. Jutel M, Blaser K, Akdis CA. Histamine receptors in immune regulation and allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2006. 26:245–259.
Article55. Mehra NK, Verduijn W, Taneja V, Drabbels J, Singh SP, Giphart MJ. Analysis of HLA-DR2-associated polymorphisms by oligonucleotide hybridization in an Asian Indian population. Hum Immunol. 1991. 32:246–253.
Article56. Texier C, Pouvelle S, Busson M, Hervé M, Charron D, Ménez A, Maillère B. HLA-DR restricted peptide candidates for bee venom immunotherapy. J Immunol. 2000. 164:3177–3184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strain-Specific Differences in House Dust Mite (Dermatophagoides farinae)-Induced Mouse Models of Allergic Rhinitis
- Relationship between Poor Immunogenicity of HLA-A2-Restricted Peptide Epitopes and Paucity of Naive CD8+ T-Cell Precursors in HLA-A2-Transgenic Mice
- Effect of DHEA Ingestion on Atopic Dermatitis-like Lesion in BALB/c Mice Sensitized by Ovalbumin
- Effect of Various Fungi on the Aflatoxin Productivity in the Culture of Asp. Flavus
- Extracellular vesicles derived from small intestinal lamina propria reduce antigen-specific immune response