Clin Exp Otorhinolaryngol.
2009 Mar;2(1):6-12. 10.3342/ceo.2009.2.1.6.
Antioxidant and Anti-Apoptotic Effect of Melatonin on the Vestibular Hair Cells of Rat Utricles
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Dankook University, Cheonan, Korea. jjking@dankook.ac.kr
- 2Medical Laser Research Center, College of Medicine, Dankook University, Cheonan, Korea.
- KMID: 1466553
- DOI: http://doi.org/10.3342/ceo.2009.2.1.6
Abstract
OBJECTIVES
Aminoglycosides are commonly used antibiotic agents, and they are known to generate free oxygen radicals within the inner ear and to cause vestibulo-cochlear toxicity and permanent damage to the sensory hair cells and neurons. Melatonin, a pineal secretory product, has the properties of being a powerful direct and indirect antioxidant. The aim of the present study was to prove the antioxidant effect of melatonin against gentamicin-induced ototoxicty.
METHODS
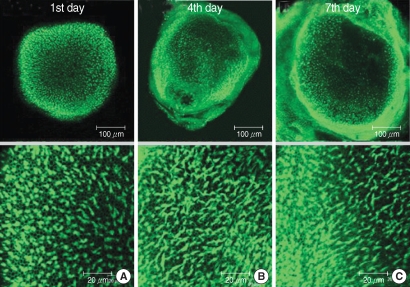
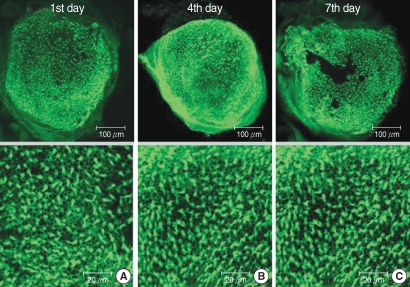
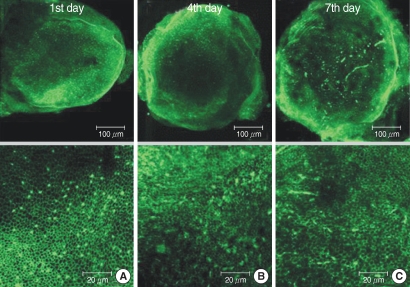
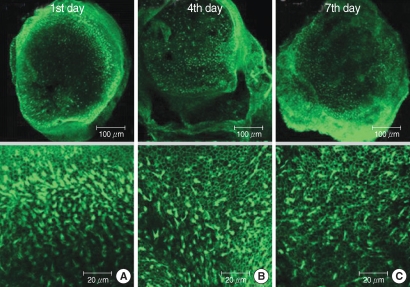
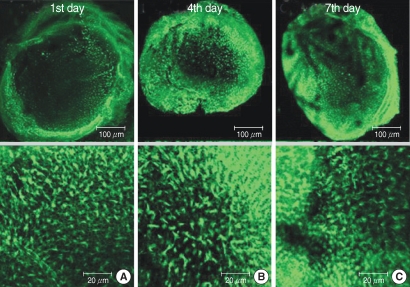
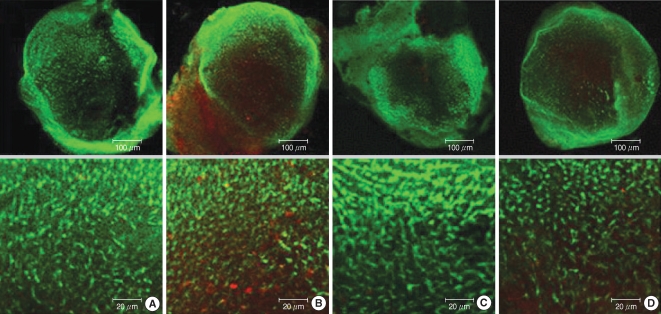
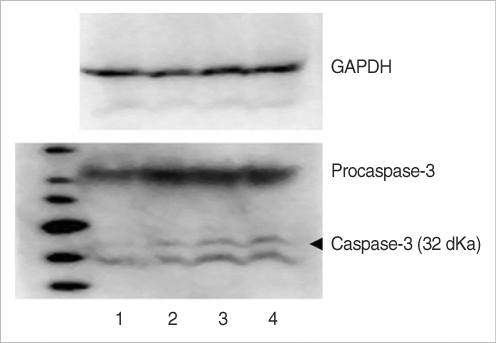
The utricular maculae of Sprague-Dawley rats were prepared from postnatal day 2-4, and these maculae were were divided into 6 groups as follows: 1) control, 2) melatonin only, 3) gentamicin only, and 4), 5), and 6) gentamicin plus melatonin (10, 50, and 100 micrometer, respectively). To count the number of hair cells, 5 utricles from each group were stained with phalloidin-FITC on the 1st, 4th, and 7th days after drug administration. Reactive oxygen species (ROS) was assessed by using the fluorescent probe hydrofluorescent diacetate acetyl ester. The caspase-3 activity was also examined with using the fluorescent caspase-3 substrate and performing Western blotting.
RESULTS
The result of this study showed that gentamicin induced the loss of utricular hair cells, and this loss of hair cells was significantly attenuated by co-administration of melatonin. Melatonin reduced ROS production and caspase-3 activation in the gentamicin treated utricular hair cells.
CONCLUSION
Our findings conclusively reveal that melatonin has protective effects against gentamicin-induced hair cell loss in the utricles of rat by inhibiting both ROS production and caspase-3 activity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Protective Effect of Hexane and Ethanol Extract of
Piper Longum L. on Gentamicin-Induced Hair Cell Loss in Neonatal Cultures
Mukesh Kumar Yadav, June Choi, Jae-Jun Song
Clin Exp Otorhinolaryngol. 2014;7(1):13-18. doi: 10.3342/ceo.2014.7.1.13.
Reference
-
1. Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995; 11. 27. 50(11):1749–1752. PMID: 8615852.
Article2. Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000; 1. 139(1-2):97–115. PMID: 10601716.
Article3. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan–Feb. 5(1):3–22. PMID: 10686428.
Article4. Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995; 10. 9(13):1277–1287. PMID: 7557017.
Article5. Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005; 7. 27(2):119–130. PMID: 16217125.
Article6. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med. 1996; 21(3):307–315. PMID: 8855441.
Article7. Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995; 10. 19(3):123–126. PMID: 8750345.
Article8. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007; 13(1):119–126. PMID: 17266591.
Article9. Reiter RJ, Acuna-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci. 2001; 6. 939:200–215. PMID: 11462772.10. Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001; 34(2):237–256. PMID: 11898866.
Article11. Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005; 10. 01. 10(19):1313–1321. PMID: 16214676.
Article12. Darlington CL, Smith PF. Vestibulotoxicity following aminoglycoside antibiotics and its prevention. Curr Opin Investig Drugs. 2003; 7. 4(7):841–846.13. Leon J, Acuna-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004; 7. 02. 75(7):765–790. PMID: 15183071.
Article14. Hardeland R, Reiter RJ, Poeggeler B, Tan DX. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993; Fall. 17(3):347–357. PMID: 8272286.
Article15. Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997; 10. 10. 278(5336):294–298. PMID: 9323209.
Article16. Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997; 8. 15. 326(Pt 1):1–16. PMID: 9337844.
Article17. Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999; 7. 23. 274(30):21155–21161. PMID: 10409669.
Article18. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004; 7. 30. 305(5684):626–629. PMID: 15286356.
Article19. Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004; 7. 60(1):89–100. PMID: 15188275.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Promotive Effect of Low Level Laser on Hair Cell Viability in Postnatal Organotypic Culture of Rat Utricles
- A Promotive Effect of Low-Level Laser on Hair Cell Regeneration Following Gentamicin Induced Ototoxicity in Postnatal Organotypic Culture of Rat Utricles
- Minocycline Protects Vestibular Hair Cells from Neomycin-Induced Ototoxicity by Inhibiting Reactive Oxygen Species Production and Caspase-3 Activity
- Preventive and Therapeutic Effects of Low Level Laser Irradiation on Gentamicin-Induced Vestibulotoxicity in Rat Utricles
- Protective Effect of Metformin on Gentamicin-Induced Vestibulotoxicity in Rat Primary Cell Culture