J Korean Surg Soc.
2009 Jul;77(1):15-28. 10.4174/jkss.2009.77.1.15.
Feasibility Assessment of Interposition Vessel Graft Substitutes in Dog Models for Later Clinical Application to Middle Hepatic Vein Reconstruction during Living Donor Liver Transplantation
- Affiliations
-
- 1Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sglee2@amc.seoul.kr
- 2Department of Anatomy and Cell Biology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1464938
- DOI: http://doi.org/10.4174/jkss.2009.77.1.15
Abstract
-
PURPOSE: Most of grafts used as interposition conduits for middle hepatic vein (MHV) in living donor liver transplantation (LDLT) have been allografts and autografts. Recently, polytetrafluoroethylene (PTFE) and bovine pericardium patch have also been used. Thus, we performed large-animal lab tests to assess the feasibility of interposition vessel graft substitutes for MHV.
METHODS
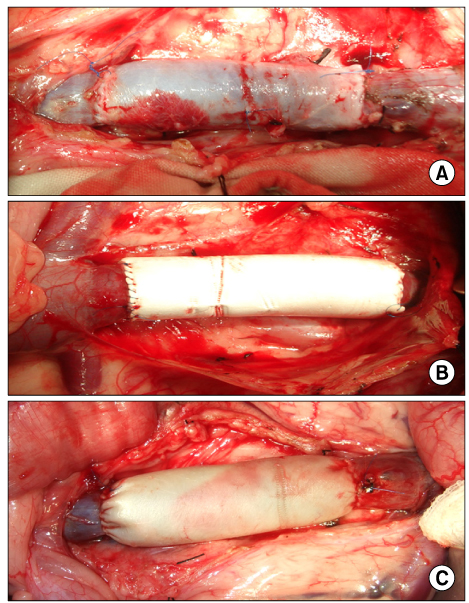
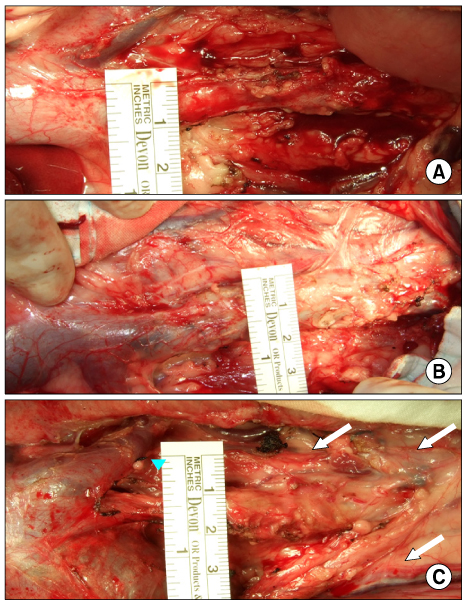
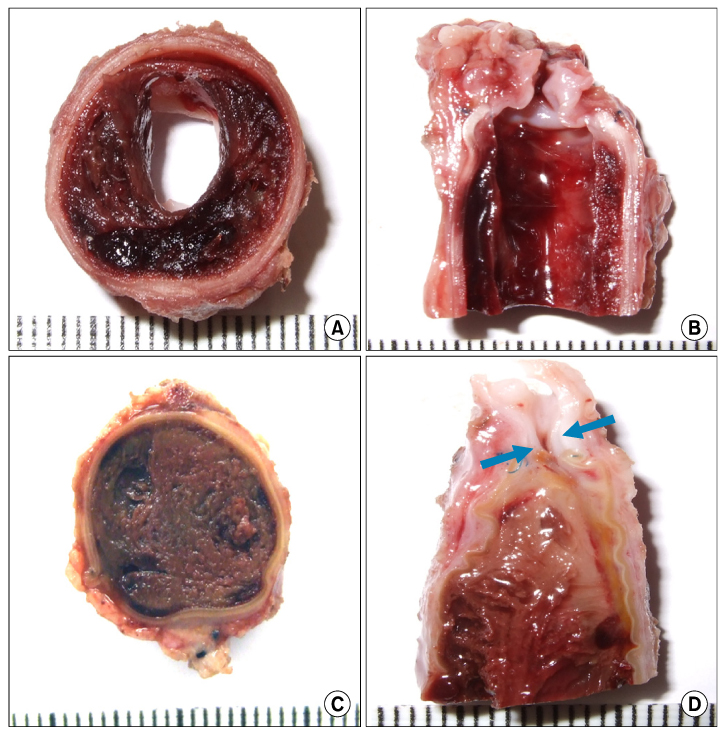
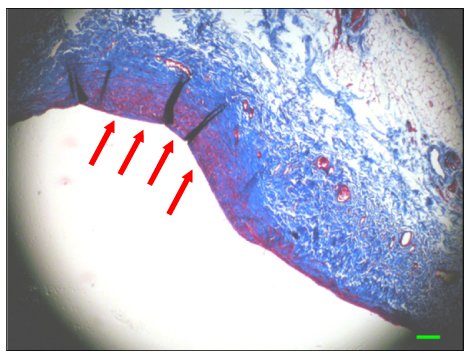
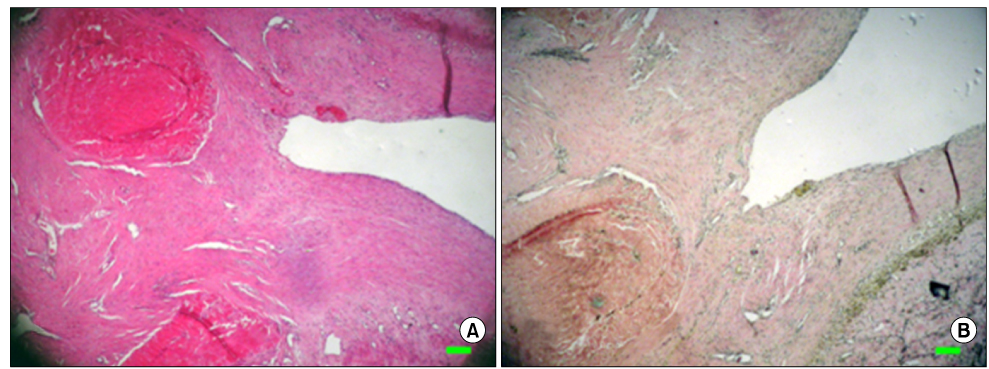
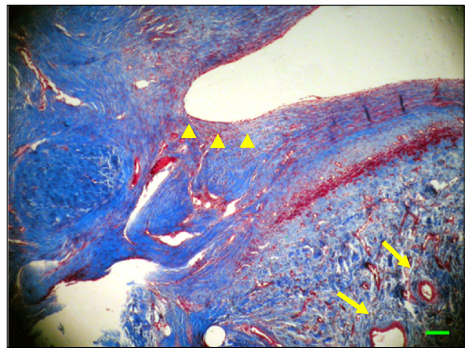
The inferior vena cava was replaced in 9 dogs with allograft (3), PTFE (3), and bovine pericardium patch (3). After 28 days, patency rate, outer and inner diameter, intimal thickness, histology, and immunohistochemistry were evaluated according to interposition grafts.
RESULTS
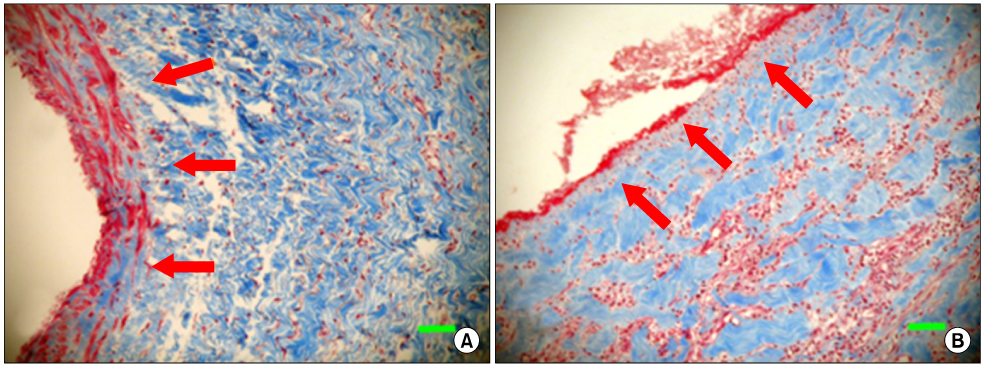
The allograft and PTFE groups were all patent at post-operative week 4, but the bovine group was not patent in all dogs. Outer diameter of anastomotic site at 4 weeks was 8.41+/-0.37, 10.83+/-0.51, and 7.41+/-0.86 mm in allograft, PTFE, and bovine group, respectively. Inner diameter of interposition graft at 4 weeks was 7.90+/-0.23, 6.33+/-0.68, and 0 mm in allograft, PTFE, and bovine groups, respectively. Intimal thickness was 48.0+/-8.6, 113.8+/-45.3, and 218.3+/-59.9microm in allograft, PTFE, and bovine groups, respectively. In histologic findings, inflammation was most severe in the bovine group. Intima of anastomotic site in the bovine group was thickest in all groups. Proliferation of smooth muscle cells was most severe in anti-alpha-actin antibody test in bovine group.
CONCLUSION
Our data implicate that the use of allografts and PTFE grafts is more feasible than bovine pericardium for MHV reconstruction in LDLT.
Keyword
MeSH Terms
-
Animals
Dogs
Glycosaminoglycans
Hepatic Veins
Humans
Immunohistochemistry
Inflammation
Liver
Liver Transplantation
Living Donors
Myocytes, Smooth Muscle
Pericardium
Polytetrafluoroethylene
Pyridines
Thiazoles
Transplantation, Homologous
Transplants
Vena Cava, Inferior
Glycosaminoglycans
Polytetrafluoroethylene
Pyridines
Thiazoles
Figure
Cited by 1 articles
-
Long-term patency and complications of ringed polytetrafluoroethylene grafts used for middle hepatic vein reconstruction in living-donor liver transplantation
I-Ji Jung, Shin Hwang, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Gil-Chun Park, Young-In Yoon, Yo-Han Park, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Sang-Hyun Kang, Sung-Gyu Lee
Korean J Transplant. 2020;34(1):31-37. doi: 10.4285/kjt.2020.34.1.31.
Reference
-
1. Lee SG, Park KM, Hwang S, Kim KH, Choi DN, Joo SH, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation. 2002. 74:54–59.2. Hwang S, Lee SG, Song GW, Lee HJ, Park JI, Ryu JH. Use of endarterectomized atherosclerotic artery allograft for hepatic vein reconstruction of living donor right lobe graft. Liver Transpl. 2007. 13:306–308.3. Yi NJ, Suh KS, Lee HW, Cho EH, Shin WY, Cho JY, et al. An artificial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl. 2007. 13:1159–1167.4. Kim BW, Wang HJ, Lee BM, Park YK, Paik OJ, Kim MW. Middle hepatic vein reconstruction of right liver graft using the glutaraldehyde-treated acellular bovine pericardium. Surgery. 2007. 141:832–834.5. Lu WD, Yu FL, Wu ZS. Superior vena cava reconstruction using bovine jugular vein conduit. Eur J Cardiothorac Surg. 2007. 32:816–817.6. Al Halees Z, Al Shahid M, Al Sanei A, Sallehuddin A, Duran C. Up to 16 years follow-up of aortic valve reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg. 2005. 28:200–205.7. Lemson MS, Tordoir JH, Daemen MJ, Kitslaar PJ. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000. 19:336–350.8. Ethridge CP, Mitchell GM, Barton RM, Morrison WA, O'Brien BM. Long microvenous allografts in rabbit femoral arteries and veins. Br J Plast Surg. 1988. 41:52–61.9. Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001. 71:812–814.10. Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, et al. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003. 237:180–185.11. McNeil PL, Muthukrishnan L, Warder E, D'Amore PA. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989. 109:811–822.12. Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991. 68:106–113.13. Davies MG, Fulton GJ, Svendsen E, Hagen PO. Time course of the regression of intimal hyperplasia in experimental vein grafts. Cardiovasc Pathol. 1999. 8:161–168.14. Sugawara Y, Makuuchi M, Tamura S, Matsui Y, Kaneko J, Hasegawa K, et al. Portal vein reconstruction in adult living donor liver transplantation using cryopreserved vein grafts. Liver Transpl. 2006. 12:1233–1236.15. Hemming AW, Reed AI. Left trisegmentectomy with reconstruction of segment 6 hepatic venous outflow using cryopreserved vein graft. J Gastrointest Surg. 2005. 9:353–356.16. Hemming AW, Reed AI, Langham MR Jr, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004. 239:712–719.17. Gloviczki P, Pairolero PC, Cherry KJ, Hallett JW Jr. Reconstruction of the vena cava and of its primary tributaries: a preliminary report. J Vasc Surg. 1990. 11:373–381.18. Gloviczki P, Pairolero PC, Toomey BJ, Bower TC, Rooke TW, Stanson AW, et al. Reconstruction of large veins for nonmalignant venous occlusive disease. J Vasc Surg. 1992. 16:750–761.19. Frost-Arner L, Bergqvist D. Effect of isovolemic hemodilution with dextran and albumin on thrombus formation in artificial vessel grafts inserted into the abdominal aorta of the rabbit. Microsurgery. 1995. 16:357–361.20. Gloviczki P, Hollier LH, Dewanjee MK, Trastek VF, Hoffman EA, Kaye MP. Experimental replacement of the inferior vena cava: factors affecting patency. Surgery. 1984. 95:657–666.21. Nardo B, Ercolani G, Montalti R, Bertelli R, Gardini A, Beltempo P, et al. Hepatic resection for primary or secondary malignancies with involvement of the inferior vena cava: is this operation safe or hazardous? J Am Coll Surg. 2005. 201:671–679.22. Del Campo C, Love J, Bowes F. Prosthetic replacement of the superior vena cava with a custom-made pericardial graft: an experimental study. Can J Surg. 1992. 35:305–309.23. Fiore AC, Brown JW, Cromartie RS, Ofstein LC, Peigh PS, Sears NS, et al. Prosthetic replacement for the thoracic vena cava: an experimental study. J Thorac Cardiovasc Surg. 1982. 84:560–568.24. Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980. 239:H14–H21.25. Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989. 105:393–400.26. Ochsner JL, Lawson JD, Eskind SJ, Mills NL, DeCamp PT. Homologous veins as an arterial substitute: long-term results. J Vasc Surg. 1984. 1:306–313.27. Augelli NV, Lupinetti FM, el Khatib H, Sanofsky SJ, Rossi NP. Allograft vein patency in a canine model. Additive effects of cryopreservation and cyclosporine. Transplantation. 1991. 52:466–470.28. Shibuya T, Kambayashi J, Okahara K, Kim DI, Kawasaki T, Sakon M, et al. Subendothelial layer of pseudointima of polytetrafluoroethylene graft is formed by transformation of fibroblasts migrated from extravascular space. Eur J Vasc Surg. 1994. 8:276–285.29. Ferrans VJ, Boyce SW, Billingham ME, Jones M, Ishihara T, Roberts WC. Calcific deposits in porcine bioprostheses: structure and pathogenesis. Am J Cardiol. 1980. 46:721–734.30. Iha K, Koja K, Kusaba A. Morphological, immunohistological and fibrinolytic features of patch grafts for reconstruction of the inferior vena cava. Cardiovasc Surg. 1994. 2:592–597.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human dermis as a new substitute for middle hepatic vein during living donor liver transplantation: early results from ongoing clinical trial
- Hepatic Vein Reconstruction for Living Donor Liver Transplantation using a Modified Right lobe Graft: Experience at Asan Medical Center and focused on Middle Hepatic Vein Reconstruction
- Standardized surgical techniques for adult living donor liver transplantation using a modified right lobe graft: a video presentation from bench to reperfusion
- Portal bifurcation reconstruction using own hepatic vein grafts due to portal vein anomaly of the living donor for the patient with portal vein thrombosis
- Hepatic artery reconstruction using interposition of autologous saphenous vein conduit for living donor liver transplantation: a case report