Korean J Bone Metab.
2012 May;19(1):35-46. 10.11005/kjbm.2012.19.1.35.
The Effects of Extracellular pH on Proliferation and Differentiation of human Bone Marrow Stem Cells
- Affiliations
-
- 1Department of Orthopedic Surgery, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea. jschang@amc.seoul.kr
- 2Nanoori Orthopedics Hospital, Seoul, Korea.
- KMID: 1464234
- DOI: http://doi.org/10.11005/kjbm.2012.19.1.35
Abstract
OBJECTIVES
The purpose of this study is to identify whether the change of pH affects the proliferation and the differentiation of human bone marrow stem cells (hBMSCs) and what mechanism is underlied.
METHODS
To achieve objective of this study, hBMSCs were cultivated in the conditioned media adjusted to potential of hydrogen (pH) ranging from 6.4 to 8.0 using addition of hydrochloric acid (HCl) and sodium hydroxide (NaOH). The ratio of proliferation of hBMSCs according to the change of pH was measured for 24 h, 48 h, and 72 h using water-soluble tetrazolium salt (WST)-8 method. To elucidate the mechanism involved, hBMSCs was subjected to blocking extracellular signal-regulated kinases (ERK) and calcium sensing receptor (CaSR) activation. The Osteogenic-related genes and alkaline phosphatase (ALP) activity were tested under the conditioned media.
RESULTS
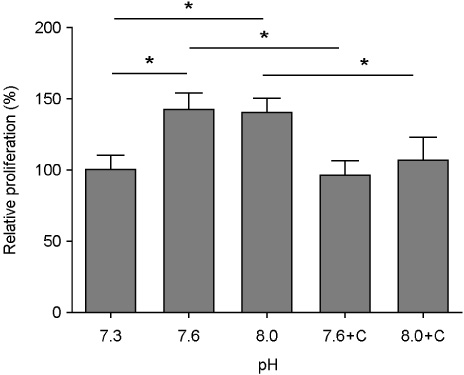
The proliferation of hBMSCs was promoted under extracellular alkali conditions (pH 7.6~8.0) via CaSR/ERK pathway. On the other hand, the differentiation was inhibited/delayed via decreased ALP activity besides gene expression at pH 8.0.
CONCLUSION
Extracellular alkali or acidic surrounding according to pH alteration can play a crucial role in hBMSC behavior including the proliferation and the differentiation.
MeSH Terms
-
Alkalies
Alkaline Phosphatase
Bone Marrow
Bone Marrow Cells
Cell Differentiation
Cell Proliferation
Culture Media, Conditioned
Extracellular Signal-Regulated MAP Kinases
Gene Expression
Hand
Humans
Hydrochloric Acid
Hydrogen
Hydrogen-Ion Concentration
Hydroxides
Receptors, Calcium-Sensing
Sodium Hydroxide
Stem Cells
Alkalies
Alkaline Phosphatase
Culture Media, Conditioned
Extracellular Signal-Regulated MAP Kinases
Hydrochloric Acid
Hydrogen
Hydroxides
Receptors, Calcium-Sensing
Sodium Hydroxide
Figure
Reference
-
1. Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAA receptors in cultured rat astrocytes. Neurosci Lett. 1991. 126:9–12.
Article2. Ramp WK, Lenz LG, Kaysinger KK. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick bones and osteoblast-like cells. Bone Miner. 1994. 24:59–73.
Article3. Reeve J, Arlot M, Wootton R, et al. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J Clin Endocrinol Metab. 1988. 66:1124–1131.
Article4. Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008. 138:415S–418S.
Article5. Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: implications for tissue engineering of bone. J Biomed Mater Res. 2002. 60:292–299.
Article6. Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res. 2003. 18:1317–1325.
Article7. Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005. 77:167–174.
Article8. Disthabanchong S, Radinahamed P, Stitchantrakul W, Hongeng S, Rajatanavin R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007. 71:201–209.
Article9. Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993. 366:575–580.
Article10. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001. 81:239–297.
Article11. Maiti A, Hait NC, Beckman MJ. Extracellular calcium-sensing receptor activation induces vitamin D receptor levels in proximal kidney HK-2G cells by a mechanism that requires phosphorylation of p38alpha MAPK. J Biol Chem. 2008. 283:175–183.
Article12. Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res. 1998. 13:1530–1538.
Article13. Yamaguchi T, Kifor O, Chattopadhyay N, Brown EM. Expression of extracellular calcium (Ca2+o)-sensing receptor in the clonal osteoblast-like cell lines, UMR-106 and SAOS-2. Biochem Biophys Res Commun. 1998. 243:753–757.
Article14. Yamaguchi T, Chattopadhyay N, Kifor O, et al. Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol. 2001. 280:C382–C393.
Article15. Chang W, Tu C, Chen TH, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999. 140:5883–5893.
Article16. Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995. 268:873–876.
Article17. Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004. 279:37241–37249.
Article18. Sugimoto T, Kanatani M, Kano J, et al. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells. J Bone Miner Res. 1993. 8:1445–1452.
Article19. Quarles LD, Hartle JE 2nd, Siddhanti SR, Guo R, Hinson TK. A distinct cation-sensing mechanism in MC3T3-E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res. 1997. 12:393–402.
Article20. Ludwig MG, Vanek M, Guerini D, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003. 425:93–98.
Article21. Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993. 18:128–131.
Article22. Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994. 4:82–89.
Article23. Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994. 19:470–473.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Duration and Magnitude of Extracellular Signal-Regulated Protein Kinase Phosphorylation Determine Adipogenesis or Osteogenesis in Human Bone Marrow-Derived Stem Cells
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Osteoblastic differentiation of adult stem cells by Biphasic Calcium Phosphate
- Enhancement of Replication and Differentiation Potential of Human Bone Marrow Stem Cells by Nicotinamide Treatment