J Clin Neurol.
2010 Dec;6(4):196-203. 10.3988/jcn.2010.6.4.196.
Measurement of Precuneal and Hippocampal Volumes Using Magnetic Resonance Volumetry in Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Dajeon, Korea.
- 2Department of Radiology, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Dajeon, Korea.
- 3Department of Neurology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Department of Neurology, Incheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Incheon, Korea.
- 5Department of Neurology, Chungnam National University Hospital, Daejeon, Korea. aelee@cnu.ac.kr
- KMID: 1462807
- DOI: http://doi.org/10.3988/jcn.2010.6.4.196
Abstract
- BACKGROUND AND PURPOSE
Alzheimer's disease (AD) is associated with structural alterations in the medial temporal lobe (MTL) and functional alterations in the posterior cortical region, especially in the early stages. However, it is unclear what mechanisms underlie these regional discrepancies or whether the posterior cortical hypometabolism reflects disconnection from the MTL lesion or is the result of local pathology. The precuneus, an area of the posteromedial cortex that is involved in the early stages of AD, has recently received a great deal of attention in functional neuroimaging studies. To assess the relationship between the precuneus and hippocampus in AD, we investigated the volumes of these two areas using a magnetic resonance volumetric method.
METHODS
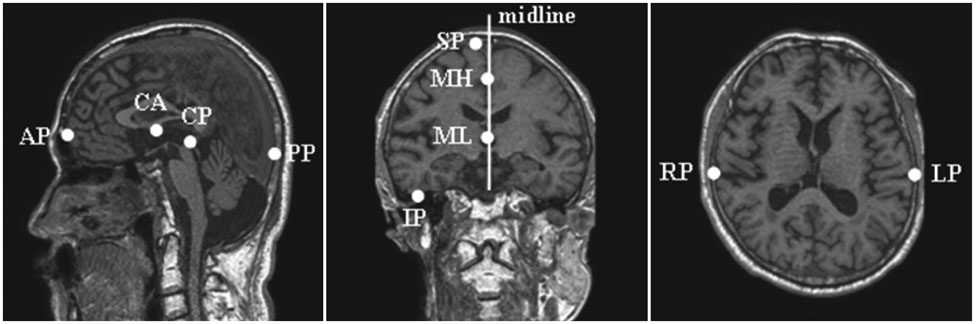
Twenty-three subjects with AD and 14 healthy age-matched controls underwent T1-weighted three-dimensional volumetric brain magnetic resonance imaging. Volumetric measurements were performed in the precuneus and hippocampus.
RESULTS
Compared to controls, AD patients exhibited a significant reduction in total precuneal volume, which was more prominent on the right side, and significant bilateral reductions in hippocampal volume. No correlation was found between the total volumes of the precuneus and hippocampus in the AD group.
CONCLUSIONS
These results suggest that volumetric measurements of both the precuneus and hippocampus are useful radiological indices for the diagnosis of AD. Furthermore, the lack of correlation is attributable to local pathology rather than being a secondary consequence of MTL pathology.
MeSH Terms
Figure
Cited by 2 articles
-
Adverse Effects of 24 Hours of Sleep Deprivation on Cognition and Stress Hormones
Eun Yeon Joo, Cindy W Yoon, Dae Lim Koo, Daeyoung Kim, Seung Bong Hong
J Clin Neurol. 2012;8(2):146-150. doi: 10.3988/jcn.2012.8.2.146.Cerebral Perfusion Changes after Acetyl-L-Carnitine Treatment in Early Alzheimer's Disease Using Single Photon Emission Computed Tomography
Hyeonseok S. Jeong, Jong-Sik Park, YoungSoon Yang, Seung-Hee Na, Yong-An Chung, In-Uk Song
Dement Neurocogn Disord. 2017;16(1):26-31. doi: 10.12779/dnd.2017.16.1.26.
Reference
-
1. Lehéricy S, Marjanska M, Mesrob L, Sarazin M, Kinkingnehun S. Magnetic resonance imaging of Alzheimer's disease. Eur Radiol. 2007. 17:347–362.
Article2. Glodzik-Sobanska L, Rusinek H, Mosconi L, Li Y, Zhan J, de Santi S, et al. The role of quantitative structural imaging in the early diagnosis of Alzheimer's disease. Neuroimaging Clin N Am. 2005. 15:803–826.
Article3. Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007. 80:S160–S167.
Article4. Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001. 71:441–447.
Article5. Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000. 47:430–439.
Article6. Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005. 10:160–184.
Article7. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 1999. 2nd ed. New York: Springer Wien New York.8. Salamon G, Salamon-Murayama N, Mongkolwat P, Russell EJ. Magnetic resonance imaging study of the parietal lobe: anatomic and radiologic correlations. Adv Neurol. 2003. 93:23–42.9. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006. 129:564–583.
Article10. Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006. 67:446–452.
Article11. Baron JC, Chételat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001. 14:298–309.
Article12. Ramani A, Jensen JH, Helpern JA. Quantitative MR imaging in Alzheimer's disease. Radiology. 2006. 241:26–44.13. Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001. 14:1238–1243.
Article14. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000. 11:805–821.
Article15. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984. 34:939–944.
Article17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. 43:2412–2414.18. Kang YW, Na DL, Hahn SH. Seoul neuropsychological screening battery. 2003. Incheon: Human Brain Research & Consulting Co..19. Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 1988. New York: Thieme Medical Publishers.20. Gath I, Geva AB. Unsupervised optimal fuzzy clustering. IEEE Trans Pattern Anal Mach Intell. 1989. 11:773–781.
Article21. Kwon MJ, Han YJ, Shin IH, Park HW. Hierarchical fuzzy segmentation of brain MR images. Int J Imaging Syst Technol. 2003. 13:115–125.
Article22. Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med. 2000. 44:973–977.
Article23. Zhou SY, Suzuki M, Takahashi T, Hagino H, Kawasaki Y, Matsui M, et al. Parietal lobe volume deficits in schizophrenia spectrum disorders. Schizophr Res. 2007. 89:35–48.
Article24. Duvernoy HM. The human hippocampus, functional anatomy, vascularisation and serial sections with MRI. 2005. 3rd ed. New York: Springer-Verlag Berlin Heidelberg.25. Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992. 42:1743–1750.
Article26. Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000. 10:752–758.
Article27. Ciumas C, Montavont A, Ryvlin P. Magnetic resonance imaging in clinical trials. Curr Opin Neurol. 2008. 21:431–436.
Article28. Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol Psychiatry. 2005. 10:147–159.
Article29. Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002. 73:657–664.
Article30. Chételat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002. 13:1939–1943.
Article31. Foundas AL, Leonard CM, Mahoney SM, Agee OF, Heilman KM. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer's disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol Behav Neurol. 1997. 10:81–89.32. Barnes J, Scahill RI, Schott JM, Frost C, Rossor MN, Fox NC. Does Alzheimer's disease affect hippocampal asymmetry? Evidence from a cross-sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord. 2005. 19:338–344.
Article33. Wolf H, Grunwald M, Kruggel F, Riedel-Heller SG, Angerhöfer S, Hojjatoleslami A, et al. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging. 2001. 22:177–186.
Article34. Rusinek H, Endo Y, De Santi S, Frid D, Tsui WH, Segal S, et al. Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology. 2004. 63:2354–2359.
Article35. Golebiowski M, Barcikowska M, Pfeffer A. Magnetic resonance imaging-based hippocampal volumetry in patients with dementia of the Alzheimer type. Dement Geriatr Cogn Disord. 1999. 10:284–288.
Article36. Ishii K, Kawachi T, Sasaki H, Kono AK, Fukuda T, Kojima Y, et al. Voxel-based morphometric comparison between early-and late-onset mild Alzheimer's disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005. 26:333–340.37. Kinkingnéhun S, Sarazin M, Lehéricy S, Guichart-Gomerz E, Hergueta T, Dubois B. VBM anticipates the rate of progression of Alzheimer disease: a 3-year longitudinal study. Neurology. 2008. 70:2201–2211.
Article38. Karas G, Scheltens P, Rombouts S, van Schijndel R, Klein M, Jones B, et al. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007. 49:967–976.
Article39. Whitwell JL, Jack CR Jr. Neuroimaging in dementia. Neurol Clin. 2007. 25:843–857.
Article40. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997. 42:85–94.
Article41. Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, et al. Current Challenges for the Early Detection of Alzheimer's Disease: Brain Imaging and CSF studies. J Clin Neurol. 2009. 5:153–166.
Article42. Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain. 2008. 131:60–71.
Article43. Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007. 68:13–19.
Article44. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009. 19:72–78.
Article45. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004. 101:4637–4642.
Article46. Villain N, Desgranges B, Viader F, de la Sayette V, Mézenge F, Landeau B, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci. 2008. 28:6174–6181.
Article47. Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005. 25:7709–7717.
Article48. Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, et al. Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009. 450:336–339.
Article49. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001. 98:676–682.
Article50. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008. 1124:1–38.51. Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004. 24:10084–10092.
Article52. Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, et al. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. J Am Geriatr Soc. 2008. 56:920–934.
Article53. Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992. 2:435–443.
Article54. Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001. 2:685–694.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Various Intracranial Volume Measurements on Hippocampal Volumetry and Modulated Voxel-based Morphometry
- Hippocampal Atrophy Identified by Volumetric Measurement in Intractable Epilepsy Cases
- The Significance and Limitation of MR Volumetry: Comparison between Normal Adults and the Patients with Epilepsy and Hippocampal Sclerosis
- Semiautomatic Three-Dimensional Threshold-Based Cardiac Computed Tomography Ventricular Volumetry in Repaired Tetralogy of Fallot: Comparison with Cardiac Magnetic Resonance Imaging
- Structural Changes in the Hippocampal Subfields in Early-Onset Mild Cognitive Impairment